Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks
- PMID: 20660616
- PMCID: PMC2916137
- DOI: 10.1084/jem.20100276
Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks
Erratum in
- J Exp Med. 2011 Aug 1;208(8):1737
Abstract
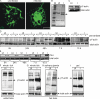
Anaplasma phagocytophilum, the agent of human anaplasmosis, persists in ticks and mammals. We show that A. phagocytophilum induces the phosphorylation of actin in an Ixodes ricinus tick cell line and Ixodes scapularis ticks, to alter the ratio of monomeric/filamentous (G/F) actin. A. phagocytophilum-induced actin phosphorylation was dependent on Ixodes p21-activated kinase (IPAK1)-mediated signaling. A. phagocytophilum stimulated IPAK1 activity via the G protein-coupled receptor Gbetagamma subunits, which mediated phosphoinositide 3-kinase (PI3K) activation. Disruption of Ixodes gbetagamma, pi3k, and pak1 reduced actin phosphorylation and bacterial acquisition by ticks. A. phagocytophilum-induced actin phosphorylation resulted in increased nuclear G actin and phosphorylated actin. The latter, in association with RNA polymerase II (RNAPII), enhanced binding of TATA box-binding protein to RNAPII and selectively promoted expression of salp16, a gene crucial for A. phagocytophilum survival. These data define a mechanism that A. phagocytophilum uses to selectively alter arthropod gene expression for its benefit and suggest new strategies to interfere with the life cycle of this intracellular pathogen, and perhaps other Rickettsia-related microbes of medical importance.
Figures




References
-
- Bell-Sakyi L., Zweygarth E., Blouin E.F., Gould E.A., Jongejan F.. 2007. Tick cell lines: tools for tick and tick-borne disease research. Trends Parasitol 23:450–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

