DNA damage signaling in response to double-strand breaks during mitosis
- PMID: 20660628
- PMCID: PMC2930281
- DOI: 10.1083/jcb.200911156
DNA damage signaling in response to double-strand breaks during mitosis
Abstract
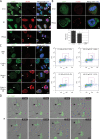
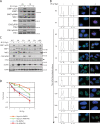
The signaling cascade initiated in response to DNA double-strand breaks (DSBs) has been extensively investigated in interphase cells. Here, we show that mitotic cells treated with DSB-inducing agents activate a "primary" DNA damage response (DDR) comprised of early signaling events, including activation of the protein kinases ataxia telangiectasia mutated (ATM) and DNA-dependent protein kinase (DNA-PK), histone H2AX phosphorylation together with recruitment of mediator of DNA damage checkpoint 1 (MDC1), and the Mre11-Rad50-Nbs1 (MRN) complex to damage sites. However, mitotic cells display no detectable recruitment of the E3 ubiquitin ligases RNF8 and RNF168, or accumulation of 53BP1 and BRCA1, at DSB sites. Accordingly, we found that DNA-damage signaling is attenuated in mitotic cells, with full DDR activation only ensuing when a DSB-containing mitotic cell enters G1. Finally, we present data suggesting that induction of a primary DDR in mitosis is important because transient inactivation of ATM and DNA-PK renders mitotic cells hypersensitive to DSB-inducing agents.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

