TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis
- PMID: 20660705
- PMCID: PMC3753007
- DOI: 10.4049/jimmunol.1000768
TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis
Abstract
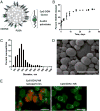
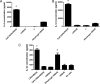
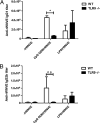
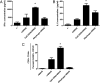
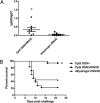
Vaccines that activate humoral and cell-mediated immune responses are urgently needed for many infectious agents, including the flaviviruses dengue and West Nile (WN) virus. Vaccine development would be greatly facilitated by a new approach, in which nanoscale modules (Ag, adjuvant, and carrier) are assembled into units that are optimized for stimulating immune responses to a specific pathogen. Toward that goal, we formulated biodegradable nanoparticles loaded with Ag and surface modified with the pathogen-associated molecular pattern CpG oligodeoxynucleotides. We chose to evaluate our construct using a recombinant envelope protein Ag from the WN virus and tested the efficiency of this system in eliciting humoral and cellular responses and providing protection against the live virus. Animals immunized with this system showed robust humoral responses polarized toward Th1 immune responses compared with predominately Th2-biased responses with the adjuvant aluminum hydroxide. Immunization with CpG oligodeoxynucleotide-modified nanoparticles resulted in a greater number of circulating effector T cells and greater activity of Ag-specific lymphocytes than unmodified nanoparticles or aluminum hydroxide. Ultimately, compared with alum, this system offered superior protection in a mouse model of WN virus encephalitis.
Figures


References
-
- Tsai TF. Flaviviruses. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Churchill Livingstone; Philadelphia, PA: 2000. pp. 1196–1206.
-
- Granwehr BP, Lillibridge KM, Higgs S, Mason PW, Aronson JF, Campbell GA, Barrett AD. West Nile virus: where are we now? Lancet Infect Dis. 2004;4:547–556. - PubMed
-
- Ng T, Hathaway D, Jennings N, Champ D, Chiang YW, Chu HJ. Equine vaccine for West Nile virus. Dev Biol (Basel) 2003;114:221–227. - PubMed
-
- Robert Putnak JBA, Coller G, Voss DW, Vaughn D, Clements I, Peters G, Bignami HS, Houng RC, Chen DA, Barvir, et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine. 2005;23:4442–4452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

