Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development
- PMID: 20660744
- PMCID: PMC2922570
- DOI: 10.1073/pnas.1009198107
Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development
Abstract
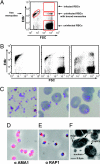
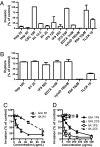
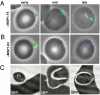
During blood-stage infection by Plasmodium falciparum, merozoites invade RBCs. Currently there is limited knowledge of cellular and molecular invasion events, and no established assays are available to readily measure and quantify invasion-inhibitory antibodies or compounds for vaccine and drug studies. We report the isolation of viable merozoites that retain their invasive capacity, at high purity and yield, purified by filtration of highly synchronous populations of schizonts. We show that the half-life of merozoite invasive capacity after rupture is 5 min at 37 degrees C, and 15 min at room temperature. Studying the kinetics of invasion revealed that 80% of invasion events occur within 10 min of mixing merozoites and RBCs. Invasion efficiency was maximum at low merozoite-to-RBC ratios and occurred efficiently in the absence of serum and with high concentrations of dialyzed nonimmune serum. We developed and optimized an invasion assay by using purified merozoites that enabled invasion-inhibitory activity of antibodies and compounds to be measured separately from other mechanisms of growth inhibition; the assay was more sensitive for detecting inhibitory activity than established growth-inhibition assays. Furthermore, with the use of purified merozoites it was possible to capture and fix merozoites at different stages of invasion for visualization by immunofluorescence microscopy and EM. We thereby demonstrate that processing of the major merozoite antigen merozoite surface protein-1 occurs at the time of RBC invasion. These findings have important implications for defining invasion events and molecular interactions, understanding immune interactions, and identifying and evaluating inhibitors to advance vaccine and drug development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Elliott SR, Beeson JG. Estimating the burden of global mortality in children aged <5 years by pathogen-specific causes. Clin Infect Dis. 2008;46:1794–1795. - PubMed
-
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. - PubMed
-
- Dowse TJ, Koussis K, Blackman MJ, Soldati-Favre D. Roles of proteases during invasion and egress by Plasmodium and Toxoplasma. Subcell Biochem. 2008;47:121–139. - PubMed
-
- Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87:377–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

