Bone marrow transplantation restores epidermal basement membrane protein expression and rescues epidermolysis bullosa model mice
- PMID: 20660747
- PMCID: PMC2922560
- DOI: 10.1073/pnas.1000044107
Bone marrow transplantation restores epidermal basement membrane protein expression and rescues epidermolysis bullosa model mice
Erratum in
- Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14514
Abstract
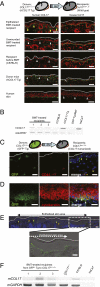
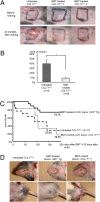
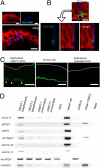
Attempts to treat congenital protein deficiencies using bone marrow-derived cells have been reported. These efforts have been based on the concepts of stem cell plasticity. However, it is considered more difficult to restore structural proteins than to restore secretory enzymes. This study aims to clarify whether bone marrow transplantation (BMT) treatment can rescue epidermolysis bullosa (EB) caused by defects in keratinocyte structural proteins. BMT treatment of adult collagen XVII (Col17) knockout mice induced donor-derived keratinocytes and Col17 expression associated with the recovery of hemidesmosomal structure and better skin manifestations, as well improving the survival rate. Both hematopoietic and mesenchymal stem cells have the potential to produce Col17 in the BMT treatment model. Furthermore, human cord blood CD34(+) cells also differentiated into keratinocytes and expressed human skin component proteins in transplanted immunocompromised (NOD/SCID/gamma(c)(null)) mice. The current conventional BMT techniques have significant potential as a systemic therapeutic approach for the treatment of human EB.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976) 2004;29:1971–1979. - PubMed
-
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. - PubMed
-
- Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed
-
- Kocher AA, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

