HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling
- PMID: 20660820
- PMCID: PMC3537327
- DOI: 10.1161/HYPERTENSIONAHA.110.152256
HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling
Erratum in
- Hypertension. 2010 Nov;56(5):e167. Jones, John E [added]
Abstract
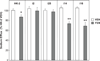
HK-2 human renal proximal tubule cells (RPTC) are commonly used in the in vitro study of "normal" RPTCs. We discovered recently that HK-2 cells are uncoupled from dopamine 1 receptor (D(1)R) adenylyl cyclase (AC) stimulation. We hypothesized that G protein-coupled receptor kinase type 4 (GRK4) single nucleotide polymorphisms may be responsible for the D(1)R/AC uncoupling in HK-2. This hypothesis was tested by genotyping GRK4 single nucleotide polymorphisms, measuring D(1)-like receptor agonist (fenoldopam)-stimulated cAMP accumulation, quantifying D(1)R inhibition of sodium transport, and testing the ability of GRK4 small interfering RNA to reverse the D(1)R/AC uncoupling. We compared HK-2 with 2 normally coupled human RPTC cell lines and 2 uncoupled RPTC cell lines. The HK-2 cell line was found to have 4 of 6 potential GRK4 single nucleotide polymorphisms known to uncouple the D(1)R from AC (namely, R65L, A142V, and A486V). AC response to fenoldopam stimulation was increased in the 2 normally coupled human RPTC cell lines (FEN: 2.02+/-0.05-fold and 2.33+/-0.19-fold over control; P<0.001; n=4) but not in the 2 uncoupled or HK-2 cell lines. GRK4 small interfering RNA rescued the fenoldopam-mediated AC stimulation in the uncoupled cells, including HK-2. The expected fenoldopam-mediated inhibition of sodium hydrogen exchanger type 3 was absent in HK-2 (n=6) and uncoupled RPTC cell lines (n=6) but was observed in the 2 normally coupled human RPTC cell lines (-25.41+/-4.7% and -27.36+/-2.70%; P<0.001; n=6), which express wild-type GRK4. Despite the fact that HK-2 cells retain many functional characteristics of RPTCs, they are not normal from the perspective of dopaminergic function.
Conflict of interest statement
Figures




References
-
- Detrisac CJ, Sens MA, Garvin AJ, Spicer SS, Sens DA. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney International. 1984;25:383–390. - PubMed
-
- Baer PC, Bereiter-Hahn J, Schubert R, Geiger H. Differentiation status of human renal proximal and distal tubular epithelial cells in vitro: Differential expression of characteristic markers. Cells Tissues Organs. 2006;184:16–22. - PubMed
-
- Wilson PD, Dillingham MA, Breckon R, Anderson RJ. Defined human renal tubular epithelia in culture: growth, characterization, and hormonal response. Am J Physiol. 1985;248:F436–F443. - PubMed
-
- Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP. Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med. 1997;129:318–329. - PubMed
-
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

