Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics
- PMID: 20661294
- PMCID: PMC2905442
- DOI: 10.1371/journal.pone.0011634
Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics
Abstract
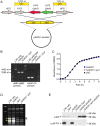
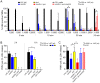
Staphylococcus aureus is a prominent human pathogen and leading cause of bacterial infection in hospitals and the community. Community-associated methicillin-resistant S. aureus (CA-MRSA) strains such as USA300 are highly virulent and, unlike hospital strains, often cause disease in otherwise healthy individuals. The enhanced virulence of CA-MRSA is based in part on increased ability to produce high levels of secreted molecules that facilitate evasion of the innate immune response. Although progress has been made, the factors that contribute to CA-MRSA virulence are incompletely defined. We analyzed the cell surface proteome (surfome) of USA300 strain LAC to better understand extracellular factors that contribute to the enhanced virulence phenotype. A total of 113 identified proteins were associated with the surface of USA300 during the late-exponential phase of growth in vitro. Protein A was the most abundant surface molecule of USA300, as indicated by combined Mascot score following analysis of peptides by tandem mass spectrometry. Unexpectedly, we identified a previously uncharacterized two-component leukotoxin-herein named LukS-H and LukF-G (LukGH)-as two of the most abundant surface-associated proteins of USA300. Rabbit antibody specific for LukG indicated it was also freely secreted by USA300 into culture media. We used wild-type and isogenic lukGH deletion strains of USA300 in combination with human PMN pore formation and lysis assays to identify this molecule as a leukotoxin. Moreover, LukGH synergized with PVL to enhance lysis of human PMNs in vitro, and contributed to lysis of PMNs after phagocytosis. We conclude LukGH is a novel two-component leukotoxin with cytolytic activity toward neutrophils, and thus potentially contributes to S. aureus virulence.
Conflict of interest statement
Figures

References
-
- King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–317. - PubMed
-
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. - PubMed
-
- Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

