Bacteriophage-resistant Staphylococcus aureus mutant confers broad immunity against staphylococcal infection in mice
- PMID: 20661301
- PMCID: PMC2908692
- DOI: 10.1371/journal.pone.0011720
Bacteriophage-resistant Staphylococcus aureus mutant confers broad immunity against staphylococcal infection in mice
Abstract
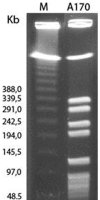
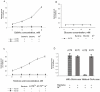
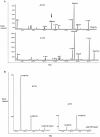
In the presence of a bacteriophage (a bacteria-attacking virus) resistance is clearly beneficial to the bacteria. As expected in such conditions, resistant bacteria emerge rapidly. However, in the absence of the phage, resistant bacteria often display reduced fitness, compared to their sensitive counterparts. The present study explored the fitness cost associated with phage-resistance as an opportunity to isolate an attenuated strain of S. aureus. The phage-resistant strain A172 was isolated from the phage-sensitive strain A170 in the presence of the M(Sa) phage. Acquisition of phage-resistance altered several properties of A172, causing reduced growth rate, under-expression of numerous genes and production of capsular polysaccharide. In vivo, A172 modulated the transcription of the TNF-alpha, IFN-gamma and Il-1beta genes and, given intramuscularly, protected mice from a lethal dose of A170 (18/20). The heat-killed vaccine also afforded protection from heterologous methicillin-resistant S. aureus (MRSA) (8/10 mice) or vancomycin-intermediate S. aureus (VISA) (9/10 mice). The same vaccine was also effective when administered as an aerosol. Anti-A172 mouse antibodies, in the dose of 10 microl/mouse, protected the animals (10/10, in two independent experiments) from a lethal dose of A170. Consisting predominantly of the sugars glucose and galactose, the capsular polysaccharide of A172, given in the dose of 25 microg/mouse, also protected the mice (20/20) from a lethal dose of A170. The above results demonstrate that selection for phage-resistance can facilitate bacterial vaccine preparation.
Conflict of interest statement
Figures


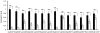

Similar articles
-
Experimental phage therapy against Staphylococcus aureus in mice.Antimicrob Agents Chemother. 2007 Aug;51(8):2765-73. doi: 10.1128/AAC.01513-06. Epub 2007 May 21. Antimicrob Agents Chemother. 2007. PMID: 17517843 Free PMC article.
-
Strain Specific Phage Treatment for Staphylococcus aureus Infection Is Influenced by Host Immunity and Site of Infection.PLoS One. 2015 Apr 24;10(4):e0124280. doi: 10.1371/journal.pone.0124280. eCollection 2015. PLoS One. 2015. PMID: 25909449 Free PMC article.
-
Recombinant PBP2a as a vaccine candidate against methicillin-resistant Staphylococcus aureus: Immunogenicity and protectivity.Microb Pathog. 2017 Jul;108:32-39. doi: 10.1016/j.micpath.2017.04.037. Epub 2017 Apr 28. Microb Pathog. 2017. PMID: 28457901
-
Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms.FEMS Microbiol Rev. 2020 Jan 1;44(1):123-153. doi: 10.1093/femsre/fuz030. FEMS Microbiol Rev. 2020. PMID: 31841134 Free PMC article. Review.
-
Overview of the risks of Staphylococcus aureus infections and their control by bacteriophages and bacteriophage-encoded products.Braz J Microbiol. 2021 Dec;52(4):2031-2042. doi: 10.1007/s42770-021-00566-4. Epub 2021 Jul 12. Braz J Microbiol. 2021. PMID: 34251609 Free PMC article. Review.
Cited by
-
Beyond antibiotics: exploring multifaceted approaches to combat bacterial resistance in the modern era: a comprehensive review.Front Cell Infect Microbiol. 2025 Mar 18;15:1493915. doi: 10.3389/fcimb.2025.1493915. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40176987 Free PMC article. Review.
-
Development of a Bacteriophage Cocktail against Pectobacterium carotovorum Subsp. carotovorum and Its Effects on Pectobacterium Virulence.Appl Environ Microbiol. 2022 Oct 11;88(19):e0076122. doi: 10.1128/aem.00761-22. Epub 2022 Sep 27. Appl Environ Microbiol. 2022. PMID: 36165651 Free PMC article.
-
Diversification of Vibrio anguillarum Driven by the Bacteriophage CHOED.Front Microbiol. 2019 Jun 20;10:1396. doi: 10.3389/fmicb.2019.01396. eCollection 2019. Front Microbiol. 2019. PMID: 31281297 Free PMC article.
-
The Rationale for Using Bacteriophage to Treat and Prevent Periprosthetic Joint Infections.Front Microbiol. 2020 Dec 21;11:591021. doi: 10.3389/fmicb.2020.591021. eCollection 2020. Front Microbiol. 2020. PMID: 33408703 Free PMC article. Review.
-
Virulence reduction in bacteriophage resistant bacteria.Front Microbiol. 2015 Apr 23;6:343. doi: 10.3389/fmicb.2015.00343. eCollection 2015. Front Microbiol. 2015. PMID: 25954266 Free PMC article.
References
-
- Marris E. Bugs gain vital ground in their battle against drugs. Nat Med. 2005;11:461. - PubMed
-
- Miller LG, Perdreau-Remington F, Rieg G, Mahdi GS, Perlroth JA, et al. Necrotising fasciitis caused by community-associated methicillin resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. - PubMed
-
- Sakoulas G, Eliopoulos GM, Fowler VG, Jr, Moellering RC, Jr, Novick R, et al. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbial protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother. 2005;49:2687–2692. - PMC - PubMed
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1988;339:520–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

