HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3
- PMID: 20661303
- PMCID: PMC2908694
- DOI: 10.1371/journal.pone.0011733
HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3
Abstract
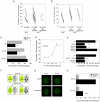
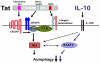
Autophagy is a homeostatic mechanism of lysosomal degradation. Defective autophagy has been linked to various disorders such as impaired control of pathogens and neurodegeneration. Autophagy is regulated by a complex array of signaling pathways that act upstream of autophagy proteins. Little is known about the role of altered regulatory signaling in disorders associated with defective autophagy. In particular, it is not known if pathogens inhibit autophagy by modulation of upstream regulatory pathways. Cells infected with HIV-1 blocked rapamycin-induced autophagy and CD40-induced autophagic killing of Toxoplasma gondii in bystander (non-HIV-1 infected) macrophage/monocytic cells. Blockade of autophagy was dependent on Src-Akt and STAT3 triggered by HIV-1 Tat and IL-10. Neutralization of the upstream receptors VEGFR, beta-integrin or CXCR4, as well as of HIV-1 Tat or IL-10 restored autophagy in macrophage/monocytic cells exposed to HIV-1-infected cells. Defective autophagic killing of T. gondii was detected in monocyte-derived macrophages from a subset of HIV-1(+) patients. This defect was also reverted by neutralization of Tat or IL-10. These studies revealed that a pathogen can impair autophagy in non-infected cells by activating counter-regulatory pathways. The fact that pharmacologic manipulation of cell signaling restored autophagy in cells exposed to HIV-1-infected cells raises the possibility of therapeutic manipulation of cell signaling to restore autophagy in HIV-1 infection.
Conflict of interest statement
Figures






Similar articles
-
Identification of Signaling Pathways by Which CD40 Stimulates Autophagy and Antimicrobial Activity against Toxoplasma gondii in Macrophages.Infect Immun. 2016 Aug 19;84(9):2616-26. doi: 10.1128/IAI.00101-16. Print 2016 Sep. Infect Immun. 2016. PMID: 27354443 Free PMC article.
-
Inhibition of Src signaling induces autophagic killing of Toxoplasma gondii via PTEN-mediated deactivation of Akt.PLoS Pathog. 2025 Jan 27;21(1):e1012907. doi: 10.1371/journal.ppat.1012907. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39869638 Free PMC article.
-
[IL-12 induces autophagy via AKT/mTOR/STAT3 signaling pathway in human hepatoma cells].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016 Jul;32(7):870-5. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016. PMID: 27363263 Chinese.
-
Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy.J Clin Virol. 2003 Feb;26(2):247-63. doi: 10.1016/s1386-6532(02)00123-3. J Clin Virol. 2003. PMID: 12600656 Review.
-
Autophagy in immunity against Toxoplasma gondii.Curr Top Microbiol Immunol. 2009;335:251-65. doi: 10.1007/978-3-642-00302-8_12. Curr Top Microbiol Immunol. 2009. PMID: 19802569 Review.
Cited by
-
Kinase AKT controls innate immune cell development and function.Immunology. 2013 Oct;140(2):143-52. doi: 10.1111/imm.12123. Immunology. 2013. PMID: 23692658 Free PMC article. Review.
-
Interactions between Autophagy and Inhibitory Cytokines.Int J Biol Sci. 2016 Jun 7;12(7):884-97. doi: 10.7150/ijbs.15194. eCollection 2016. Int J Biol Sci. 2016. PMID: 27313501 Free PMC article. Review.
-
HIV Infection, Chromosome Instability, and Micronucleus Formation.Viruses. 2023 Jan 4;15(1):155. doi: 10.3390/v15010155. Viruses. 2023. PMID: 36680195 Free PMC article. Review.
-
Autophagy inducer rapamycin treatment reduces IFN-I-mediated Inflammation and improves anti-HIV-1 T cell response in vivo.JCI Insight. 2022 Nov 22;7(22):e159136. doi: 10.1172/jci.insight.159136. JCI Insight. 2022. PMID: 36509289 Free PMC article.
-
Neuroinflammation & pre-mature aging in the context of chronic HIV infection and drug abuse: Role of dysregulated autophagy.Brain Res. 2019 Dec 1;1724:146446. doi: 10.1016/j.brainres.2019.146446. Epub 2019 Sep 12. Brain Res. 2019. PMID: 31521638 Free PMC article. Review.
References
-
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. - PubMed
-
- Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Comm. 2004;313:453–458. - PubMed
-
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MC, et al. Autophagy is defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. - PubMed
-
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, et al. Autophagy defends cells against invading Group A Streptococcus. Science. 2004;306:1037–1040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

