Suppressed expression of type 2 3alpha/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma
- PMID: 20661409
- PMCID: PMC2907123
Suppressed expression of type 2 3alpha/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma
Abstract
The diagnosis of endometrial hyperplasia and endometrial type adenocarcinoma arising within the uterine cavity has long been rested on morphologic criteria. Although distinction between normal endometrial epithelium from adenocarcinoma is usually straightforward, the separation between normal and hyperplastic endometrium, particularly those cases without atypia, can be a diagnostic challenge. The same is true in separation of hyperplastic endometrium with atypia from endometrial-type endometrial adenocarcinoma. Type 2 3alpha-/type 5 17beta-hydroxysteroid dehydrogenase (HSD) (AKR1C3) is a multifunctional enzyme involved in androgen, estrogen, progesterone, and pros-taglandin metabolism. Its expression has been shown in the epithelium of the renal tubules, urothelial epithelium, and endothelial cells in normal tissues as well as in prostatic adenocarcinoma. The proliferation and maintenance of endometrial epithelium is dependent on both estrogen and progesterone; and AKR1C3-mediated steroid metabolism may play a critical role in the maintenance of viable normal and abnormal endometrial epithelium. We studied the expression of AKR1C3 in 33 endometrial biopsy specimens including 13 cases of normal proliferative endometrium, 8 cases of hyperplastic endometrium with and without atypia, and 12 cases of primary endometrial adenocarcinoma of endometrial type. We demonstrated a uniform, diffuse, and strong expression of AKR1C3 in normal endometrial epithelium but not in endometrial stromal cells. In contrast, the expression of AKR1C3 is reduced in both hyperplastic and carcinomatous endometrial epithelium. These findings suggest that AKR1C3 may play important roles in the physiology of endometrial cells and that suppressed AKR1C3 expression may represent a feature that allows differentiation of hyperplastic and neoplastic endometrial epithelium from normal endometrial epithelium. However, reduced AKR1C3 expression cannot distinguish hyperplastic endometrium from endometrial adenocarcinoma of endometrial type. The biologic and pathological roles of AKR1C3 in endometrial epithelium require further investigation.
Keywords: Aldo-keto reductase; endomtrial cancer; estrogen; progesterone; prostaglandin.
Figures
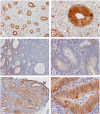
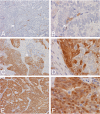
Similar articles
-
Aldo-keto reductase family 1 member C3 (AKR1C3) is expressed in adenocarcinoma and squamous cell carcinoma but not small cell carcinoma.Int J Clin Exp Pathol. 2012;5(4):278-89. Epub 2012 Apr 26. Int J Clin Exp Pathol. 2012. PMID: 22670171 Free PMC article.
-
Differential expression of type 2 3α/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) in tumors of the central nervous system.Int J Clin Exp Pathol. 2010 Mar 25;3(8):743-54. Int J Clin Exp Pathol. 2010. PMID: 21151387 Free PMC article.
-
Increased expression of type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma.Endocr Relat Cancer. 2006 Mar;13(1):169-80. doi: 10.1677/erc.1.01048. Endocr Relat Cancer. 2006. PMID: 16601286
-
Structural basis of the multispecificity demonstrated by 17beta-hydroxysteroid dehydrogenase types 1 and 5.Mol Cell Endocrinol. 2006 Mar 27;248(1-2):38-46. doi: 10.1016/j.mce.2005.11.035. Epub 2006 Feb 15. Mol Cell Endocrinol. 2006. PMID: 16480815 Review.
-
AKR1C3 in carcinomas: from multifaceted roles to therapeutic strategies.Front Pharmacol. 2024 Mar 8;15:1378292. doi: 10.3389/fphar.2024.1378292. eCollection 2024. Front Pharmacol. 2024. PMID: 38523637 Free PMC article. Review.
Cited by
-
Enzymes of the AKR1B and AKR1C Subfamilies and Uterine Diseases.Front Pharmacol. 2012 Mar 13;3:34. doi: 10.3389/fphar.2012.00034. eCollection 2012. Front Pharmacol. 2012. PMID: 22419909 Free PMC article.
-
Androgens in endometrial carcinoma: the killer or helper?J Endocrinol Invest. 2023 Mar;46(3):457-464. doi: 10.1007/s40618-022-01916-1. Epub 2022 Dec 30. J Endocrinol Invest. 2023. PMID: 36583833 Free PMC article. Review.
-
Upregulation of AKR1C3 in Sodium Butyrate Treated G361 Cell.Ann Dermatol. 2023 Dec;35(6):468-471. doi: 10.5021/ad.22.108. Ann Dermatol. 2023. PMID: 38086362 Free PMC article. No abstract available.
-
Aldo-keto reductase family 1 member C3 (AKR1C3) is expressed in adenocarcinoma and squamous cell carcinoma but not small cell carcinoma.Int J Clin Exp Pathol. 2012;5(4):278-89. Epub 2012 Apr 26. Int J Clin Exp Pathol. 2012. PMID: 22670171 Free PMC article.
-
Elevated AKR1C3 expression promotes prostate cancer cell survival and prostate cell-mediated endothelial cell tube formation: implications for prostate cancer progression.BMC Cancer. 2010 Dec 6;10:672. doi: 10.1186/1471-2407-10-672. BMC Cancer. 2010. PMID: 21134280 Free PMC article.
References
-
- Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma–new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54:667–679. - PubMed
-
- Berstein LM, Tchernobrovkina AE, Gamajunova VB, Kovalevskij AJ, Vasilyev DA, Chepik OF, Turkevitch EA, Tsyrlina EV, Maximov SJ, Ashrafian LA, Thijssen JH. Tumor estrogen content and clinico-morphological and endocrine features of endometrial cancer. J Cancer Res Clin Oncol. 2003;129:245–249. - PubMed
-
- Legro RS, Kunselman AR, Miller SA, Satyaswaroop PG. Role of androgens in the growth of endometrial carcinoma: an in vivo animal model. Am J Obstet Gynecol. 2001;184:303–308. - PubMed
-
- Voigt LF, Weiss NS, Chu J, Daling JR, McKnight B, van Belle G. Progestagen supplementation of exogenous oestrogens and risk of endometrial cancer. Lancet. 1991;338:274–277. - PubMed
-
- Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam EW, Brosens JJ. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
