Epigenetic analysis of KSHV latent and lytic genomes
- PMID: 20661424
- PMCID: PMC2908616
- DOI: 10.1371/journal.ppat.1001013
Epigenetic analysis of KSHV latent and lytic genomes
Abstract
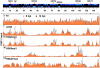
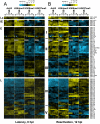
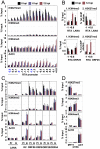
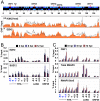
Epigenetic modifications of the herpesviral genome play a key role in the transcriptional control of latent and lytic genes during a productive viral lifecycle. In this study, we describe for the first time a comprehensive genome-wide ChIP-on-Chip analysis of the chromatin associated with the Kaposi's sarcoma-associated herpesvirus (KSHV) genome during latency and lytic reactivation. Depending on the gene expression class, different combinations of activating [acetylated H3 (AcH3) and H3K4me3] and repressive [H3K9me3 and H3K27me3] histone modifications are associated with the viral latent genome, which changes upon reactivation in a manner that is correlated with their expression. Specifically, both the activating marks co-localize on the KSHV latent genome, as do the repressive marks. However, the activating and repressive histone modifications are mutually exclusive of each other on the bulk of the latent KSHV genome. The genomic region encoding the IE genes ORF50 and ORF48 possesses the features of a bivalent chromatin structure characterized by the concomitant presence of the activating H3K4me3 and the repressive H3K27me3 marks during latency, which rapidly changes upon reactivation with increasing AcH3 and H3K4me3 marks and decreasing H3K27me3. Furthermore, EZH2, the H3K27me3 histone methyltransferase of the Polycomb group proteins (PcG), colocalizes with the H3K27me3 mark on the entire KSHV genome during latency, whereas RTA-mediated reactivation induces EZH2 dissociation from the genomic regions encoding IE and E genes concurrent with decreasing H3K27me3 level and increasing IE/E lytic gene expression. Moreover, either the inhibition of EZH2 expression by a small molecule inhibitor DZNep and RNAi knockdown, or the expression of H3K27me3-specific histone demethylases apparently induced the KSHV lytic gene expression cascade. These data indicate that histone modifications associated with the KSHV latent genome are involved in the regulation of latency and ultimately in the control of the temporal and sequential expression of the lytic gene cascade. In addition, the PcG proteins play a critical role in the control of KSHV latency by maintaining a reversible heterochromatin on the KSHV lytic genes. Thus, the regulation of the spatial and temporal association of the PcG proteins with the KSHV genome may be crucial for propagating the KSHV lifecycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. - PubMed
-
- MacDonald VE, Howe LJ. Histone acetylation: where to go and how to get there. Epigenetics. 2009;4:139–143. - PubMed
-
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

