Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors
- PMID: 20661446
- PMCID: PMC2908686
- DOI: 10.1371/journal.pone.0011717
Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors
Abstract
Background: Tetratricopeptide repeat (TPR) motif containing co-chaperones of the chaperone Hsp90 are considered control modules that govern activity and specificity of this central folding platform. Steroid receptors are paradigm clients of Hsp90. The influence of some TPR proteins on selected receptors has been described, but a comprehensive analysis of the effects of TPR proteins on all steroid receptors has not been accomplished yet.
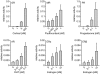
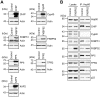
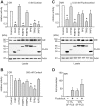
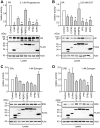
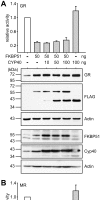
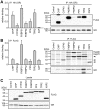
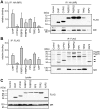
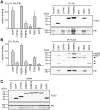
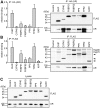
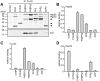
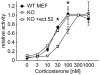
Methodology and principal findings: We compared the influence of the TPR proteins FK506 binding proteins 51 and 52, protein phosphatase-5, C-terminus of Hsp70 interacting protein, cyclophillin 40, hepatitis-virus-B X-associated protein-2, and tetratricopeptide repeat protein-2 on all six steroid hormone receptors in a homogeneous mammalian cell system. To be able to assess each cofactor's effect on the transcriptional activity of on each steroid receptor we employed transient transfection in a reporter gene assay. In addition, we evaluated the interactions of the TPR proteins with the receptors and components of the Hsp90 chaperone heterocomplex by coimmunoprecipitation. In the functional assays, corticosteroid and progesterone receptors displayed the most sensitive and distinct reaction to the TPR proteins. Androgen receptor's activity was moderately impaired by most cofactors, whereas the Estrogen receptors' activity was impaired by most cofactors only to a minor degree. Second, interaction studies revealed that the strongly receptor-interacting co-chaperones were all among the inhibitory proteins. Intriguingly, the TPR-proteins also differentially co-precipitated the heterochaperone complex components Hsp90, Hsp70, and p23, pointing to differences in their modes of action.
Conclusion and significance: The results of this comprehensive study provide important insight into chaperoning of diverse client proteins via the combinatorial action of (co)-chaperones. The differential effects of the TPR proteins on steroid receptors bear on all physiological processes related to steroid hormone activity.
Conflict of interest statement
Figures
Similar articles
-
Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore.Nucleus. 2010 Jul-Aug;1(4):299-308. doi: 10.4161/nucl.1.4.11743. Nucleus. 2010. PMID: 21113270 Free PMC article.
-
The cochaperone SGTA (small glutamine-rich tetratricopeptide repeat-containing protein alpha) demonstrates regulatory specificity for the androgen, glucocorticoid, and progesterone receptors.J Biol Chem. 2014 May 30;289(22):15297-308. doi: 10.1074/jbc.M113.535229. Epub 2014 Apr 21. J Biol Chem. 2014. PMID: 24753260 Free PMC article.
-
Interaction of the Hsp90 cochaperone cyclophilin 40 with Hsc70.Cell Stress Chaperones. 2004 Summer;9(2):167-81. doi: 10.1379/csc-26r.1. Cell Stress Chaperones. 2004. PMID: 15497503 Free PMC article.
-
Steroid receptor interactions with heat shock protein and immunophilin chaperones.Endocr Rev. 1997 Jun;18(3):306-60. doi: 10.1210/edrv.18.3.0303. Endocr Rev. 1997. PMID: 9183567 Review.
-
Therapeutic Targeting of the FKBP52 Co-Chaperone in Steroid Hormone Receptor-Regulated Physiology and Disease.Curr Mol Pharmacol. 2015;9(2):109-25. doi: 10.2174/1874467208666150519114115. Curr Mol Pharmacol. 2015. PMID: 25986565 Review.
Cited by
-
The roles of co-chaperone CCRP/DNAJC7 in Cyp2b10 gene activation and steatosis development in mouse livers.PLoS One. 2014 Dec 26;9(12):e115663. doi: 10.1371/journal.pone.0115663. eCollection 2014. PLoS One. 2014. PMID: 25542016 Free PMC article.
-
Hsp90 and FKBP51: complex regulators of psychiatric diseases.Philos Trans R Soc Lond B Biol Sci. 2018 Jan 19;373(1738):20160532. doi: 10.1098/rstb.2016.0532. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29203717 Free PMC article. Review.
-
The helix 1-3 loop in the glucocorticoid receptor LBD is a regulatory element for FKBP cochaperones.Mol Endocrinol. 2013 Jul;27(7):1020-35. doi: 10.1210/me.2012-1023. Epub 2013 May 17. Mol Endocrinol. 2013. PMID: 23686112 Free PMC article.
-
GR Utilizes a Co-Chaperone Cytoplasmic CAR Retention Protein to Form an N/C Interaction.Nucl Recept Signal. 2018 Oct 24;15:1550762918801072. doi: 10.1177/1550762918801072. eCollection 2018. Nucl Recept Signal. 2018. PMID: 30718983 Free PMC article.
-
Organization and function of the FKBP52 and FKBP51 genes.Curr Opin Pharmacol. 2011 Aug;11(4):308-13. doi: 10.1016/j.coph.2011.03.013. Epub 2011 Apr 21. Curr Opin Pharmacol. 2011. PMID: 21514887 Free PMC article. Review.
References
-
- Katzenellenbogen JA, O'Malley BW, Katzenellenbogen BS. Tripartite steroid hormone receptor pharmacology: interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol Endocrinol. 1996;10:119–131. - PubMed
-
- Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. - PubMed
-
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. - PubMed
-
- Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? 178. Cell. 1998;93:487–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

