Six principles of visual cortical dynamics
- PMID: 20661451
- PMCID: PMC2906257
- DOI: 10.3389/fnsys.2010.00028
Six principles of visual cortical dynamics
Abstract
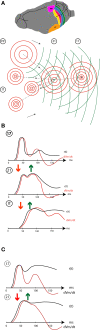
A fundamental goal in vision science is to determine how many neurons in how many areas are required to compute a coherent interpretation of the visual scene. Here I propose six principles of cortical dynamics of visual processing in the first 150 ms following the appearance of a visual stimulus. Fast synaptic communication between neurons depends on the driving neurons and the biophysical history and driving forces of the target neurons. Under these constraints, the retina communicates changes in the field of view driving large populations of neurons in visual areas into a dynamic sequence of feed-forward communication and integration of the inward current of the change signal into the dendrites of higher order area neurons (30-70 ms). Simultaneously an even larger number of neurons within each area receiving feed-forward input are pre-excited to sub-threshold levels. The higher order area neurons communicate the results of their computations as feedback adding inward current to the excited and pre-excited neurons in lower areas. This feedback reconciles computational differences between higher and lower areas (75-120 ms). This brings the lower area neurons into a new dynamic regime characterized by reduced driving forces and sparse firing reflecting the visual areas interpretation of the current scene (140 ms). The population membrane potentials and net-inward/outward currents and firing are well behaved at the mesoscopic scale, such that the decoding in retinotopic cortical space shows the visual areas' interpretation of the current scene. These dynamics have plausible biophysical explanations. The principles are theoretical, predictive, supported by recent experiments and easily lend themselves to experimental tests or computational modeling.
Keywords: cortical theory; feedback; inter-area communication; laminar firing; membrane potential; object motion; object vision; voltage-sensitive dyes.
Figures





Similar articles
-
Laminar firing and membrane dynamics in four visual areas exposed to two objects moving to occlusion.Front Syst Neurosci. 2013 Jun 25;7:23. doi: 10.3389/fnsys.2013.00023. eCollection 2013. Front Syst Neurosci. 2013. PMID: 23805082 Free PMC article.
-
Cortical Membrane Potential Dynamics and Laminar Firing during Object Motion.Front Syst Neurosci. 2009 Aug 27;3:7. doi: 10.3389/neuro.06.007.2009. eCollection 2009. Front Syst Neurosci. 2009. PMID: 19753323 Free PMC article.
-
Columnar processing of border ownership in primate visual cortex.Elife. 2021 Nov 30;10:e72573. doi: 10.7554/eLife.72573. Elife. 2021. PMID: 34845986 Free PMC article.
-
Cortical Specializations Underlying Fast Computations.Neuroscientist. 2016 Apr;22(2):145-64. doi: 10.1177/1073858415571539. Epub 2015 Feb 17. Neuroscientist. 2016. PMID: 25689988 Free PMC article. Review.
-
Mind the last spike - firing rate models for mesoscopic populations of spiking neurons.Curr Opin Neurobiol. 2019 Oct;58:155-166. doi: 10.1016/j.conb.2019.08.003. Epub 2019 Oct 4. Curr Opin Neurobiol. 2019. PMID: 31590003 Review.
Cited by
-
Visual processing in rapid-chase systems: image processing, attention, and awareness.Front Psychol. 2011 Jul 15;2:169. doi: 10.3389/fpsyg.2011.00169. eCollection 2011. Front Psychol. 2011. PMID: 21811484 Free PMC article.
-
Early recurrent feedback facilitates visual object recognition under challenging conditions.Front Psychol. 2014 Jul 1;5:674. doi: 10.3389/fpsyg.2014.00674. eCollection 2014. Front Psychol. 2014. PMID: 25071647 Free PMC article.
-
Rapid processing of closure and viewpoint-invariant symmetry: behavioral criteria for feedforward processing.Psychol Res. 2014 Jan;78(1):37-54. doi: 10.1007/s00426-013-0478-8. Epub 2013 Feb 13. Psychol Res. 2014. PMID: 23404712
-
Independent predictors of neuronal adaptation in human primary visual cortex measured with high-gamma activity.Neuroimage. 2012 Jan 16;59(2):1639-46. doi: 10.1016/j.neuroimage.2011.09.014. Epub 2011 Sep 16. Neuroimage. 2012. PMID: 21945696 Free PMC article.
-
Laminar firing and membrane dynamics in four visual areas exposed to two objects moving to occlusion.Front Syst Neurosci. 2013 Jun 25;7:23. doi: 10.3389/fnsys.2013.00023. eCollection 2013. Front Syst Neurosci. 2013. PMID: 23805082 Free PMC article.
References
-
- Arieli A., Shoham D., Hildesheim R., Grinvald A. (1995). Coherent spatiotemporal patterns of ongoing activity revealed by real-time optical imaging coupled with single-unit recording in the cat visual cortex. J. Neurophysiol. 73, 2072–2093 - PubMed
LinkOut - more resources
Full Text Sources

