Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg)
- PMID: 20661468
- PMCID: PMC2908536
- DOI: 10.1371/journal.pone.0011469
Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg)
Abstract
Background: Tumor-derived microvesicles (TMV) or exosomes are present in body fluids of patients with cancer and might be involved in tumor progression. The frequency and suppressor functions of peripheral blood CD4(+)CD25(high)FOXP3(+) Treg are higher in patients with cancer than normal controls. The hypothesis is tested that TMV contribute to induction/expansion/and activation of human Treg.
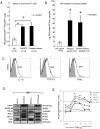
Methodology/principal findings: TMV isolated from supernatants of tumor cells but not normal cells induced the generation and enhanced expansion of human Treg. TMV also mediated conversion of CD4(+)CD25(neg) T cells into CD4(+)CD25(high)FOXP3(+) Treg. Upon co-incubation with TMV, Treg showed an increased FasL, IL-10, TGF-beta1, CTLA-4, granzyme B and perforin expression (p<0.05) and mediated stronger suppression of responder cell (RC) proliferation (p<0.01). Purified Treg were resistant to TMV-mediated apoptosis relative to other T cells. TMV also increased phospho-SMAD2/3 and phospho-STAT3 expression in Treg. Neutralizing Abs specific for TGF-beta1 and/or IL-10 significantly inhibited TMV ability to expand Treg.
Conclusions/significance: This study suggests that TMV have immunoregulatory properties. They induce Treg, promote Treg expansion, up-regulate Treg suppressor function and enhance Treg resistance to apoptosis. Interactions of TMV with Treg represent a newly-defined mechanism that might be involved in regulating peripheral tolerance by tumors and in supporting immune evasion of human cancers.
Conflict of interest statement
Figures






References
-
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. - PubMed
-
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. - PubMed
-
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

