High-level expression and purification of Cys-loop ligand-gated ion channels in a tetracycline-inducible stable mammalian cell line: GABAA and serotonin receptors
- PMID: 20662008
- PMCID: PMC2975136
- DOI: 10.1002/pro.456
High-level expression and purification of Cys-loop ligand-gated ion channels in a tetracycline-inducible stable mammalian cell line: GABAA and serotonin receptors
Abstract
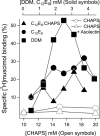
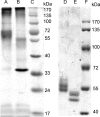
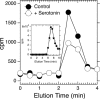
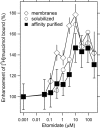
The human neuronal Cys-loop ligand-gated ion channel superfamily of ion channels are important determinants of human behavior and the target of many drugs. It is essential for their structural characterization to achieve high-level expression in a functional state. The aim of this work was to establish stable mammalian cell lines that enable high-level heterologous production of pure receptors in a state that supports agonist-induced allosteric conformational changes. In a tetracycline-inducible stable human embryonic kidney cells (HEK293S) cell line, GABA(A) receptors containing α1 and β3 subunits could be expressed with specific activities of 29-34 pmol/mg corresponding to 140-170 pmol/plate, the highest expression level reported so far. Comparable figures for serotonin (5-HT(3A)) receptors were 49-63 pmol/mg and 245-315 pmol/plate. The expression of 10 nmol of either receptor in suspension in a bioreactor required 0.3-3.0 L. Both receptor constructs had a FLAG epitope inserted at the N-terminus and could be purified in one step after solubilization using ANTI-FLAG affinity chromatography with yields of 30-40%. Purified receptors were functional. Binding of the agonist [(3)H]muscimol to the purified GABA(A)R was enhanced allosterically by the general anesthetic etomidate, and purified 5-hydroxytryptamine-3A receptor supported serotonin-stimulated cation flux when reconstituted into lipid vesicles.
Copyright © 2010 The Protein Society.
Figures
Similar articles
-
Human α1β3γ2L gamma-aminobutyric acid type A receptors: High-level production and purification in a functional state.Protein Sci. 2014 Feb;23(2):157-66. doi: 10.1002/pro.2401. Epub 2013 Dec 16. Protein Sci. 2014. PMID: 24288268 Free PMC article.
-
Expression and purification of a functional heteromeric GABAA receptor for structural studies.PLoS One. 2018 Jul 20;13(7):e0201210. doi: 10.1371/journal.pone.0201210. eCollection 2018. PLoS One. 2018. PMID: 30028870 Free PMC article.
-
Discovery of a novel allosteric modulator of 5-HT3 receptors: inhibition and potentiation of Cys-loop receptor signaling through a conserved transmembrane intersubunit site.J Biol Chem. 2012 Jul 20;287(30):25241-54. doi: 10.1074/jbc.M112.360370. Epub 2012 May 15. J Biol Chem. 2012. PMID: 22589534 Free PMC article.
-
The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function.Biochem Soc Trans. 2004 Jun;32(Pt3):529-34. doi: 10.1042/BST0320529. Biochem Soc Trans. 2004. PMID: 15157178 Review.
-
The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review).Mol Membr Biol. 2002 Jan-Mar;19(1):11-26. doi: 10.1080/09687680110110048. Mol Membr Biol. 2002. PMID: 11989819 Review.
Cited by
-
Druggable Lipid Binding Sites in Pentameric Ligand-Gated Ion Channels and Transient Receptor Potential Channels.Front Physiol. 2022 Jan 4;12:798102. doi: 10.3389/fphys.2021.798102. eCollection 2021. Front Physiol. 2022. PMID: 35069257 Free PMC article. Review.
-
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels.Can J Anaesth. 2011 Feb;58(2):191-205. doi: 10.1007/s12630-010-9419-9. Epub 2011 Jan 7. Can J Anaesth. 2011. PMID: 21213095 Free PMC article. Review.
-
Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [³H]TDBzl-etomidate, a photoreactive etomidate analogue.Biochemistry. 2012 Jan 31;51(4):836-47. doi: 10.1021/bi201772m. Epub 2012 Jan 23. Biochemistry. 2012. PMID: 22243422 Free PMC article.
-
p-(4-Azipentyl)propofol: a potent photoreactive general anesthetic derivative of propofol.J Med Chem. 2011 Dec 8;54(23):8124-35. doi: 10.1021/jm200943f. Epub 2011 Nov 10. J Med Chem. 2011. PMID: 22029276 Free PMC article.
-
A potent photoreactive general anesthetic with novel binding site selectivity for GABAA receptors.Eur J Med Chem. 2020 May 15;194:112261. doi: 10.1016/j.ejmech.2020.112261. Epub 2020 Mar 23. Eur J Med Chem. 2020. PMID: 32247113 Free PMC article.
References
-
- Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. - PubMed
-
- Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci. 2010;31:161–174. - PubMed
-
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

