Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis
- PMID: 20662061
- PMCID: PMC2970687
- DOI: 10.1002/art.27667
Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis
Abstract
Objective: The concept that intraarticular crystals of uric acid by themselves trigger episodes of painful gouty arthritis is inconsistent with the clinical reality. Patients with large deposits of monosodium urate monohydrate (MSU) crystals (tophi) do not necessarily experience gouty attacks. In fact, it is the excessive consumption of food or alcohol that elicits the inflammation of the acute gout attack. The aim of this study was to identify the precise mechanism that initiates flares of gouty arthritis.
Methods: Human peripheral blood mononuclear cells (PBMCs) and murine macrophages were stimulated in vitro with MSU, free fatty acids (FFAs), or both in combination. Thereafter, production of interleukin-1β (IL-1β) and activation of caspase 1 were determined. Gouty arthritis was induced in mice with deficiencies in the genes for caspase 1, ASC, NALP3, or IL-1β, and the lack of inflammasome activity during joint swelling or other joint pathologic features was investigated in these mice.
Results: MSU crystals had no biologic effects on PBMCs from healthy subjects, whereas the FFA C18:0 in the presence of MSU crystals induced the release of large amounts of IL-1β following engagement of Toll-like receptor 2 (TLR-2). Interaction of FFAs, but not alcohol, with TLR-2 synergized with MSU crystals to induce an inflammatory reaction. An important event of MSU/FFA-induced acute joint inflammation is the activation of the inflammasome. MSU/FFA-induced release of IL-1β was dependent on activation of caspase 1 and ASC, but surprisingly, not NALP3.
Conclusion: The synergistic effect between FFAs and MSU crystals leads to ASC/caspase 1-driven IL-1β release. This mechanism could explain how constitutionally derived metabolic events initiate attacks of gout via the induction of IL-1β-mediated joint inflammation.
Copyright © 2010 by the American College of Rheumatology.
Figures


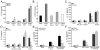
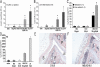


Comment in
-
How interleukin-1β induces gouty arthritis.Arthritis Rheum. 2010 Nov;62(11):3140-4. doi: 10.1002/art.27663. Arthritis Rheum. 2010. PMID: 20662058 Free PMC article. No abstract available.
References
-
- Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–8. - PubMed
-
- Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56:3183–8. - PubMed
-
- Tramontini N, Huber C, Liu-Bryan R, Terkeltaub RA, Kilgore KS. Central role of complement membrane attack complex in monosodium urate crystal-induced neutrophilic rabbit knee synovitis. Arthritis Rheum. 2004;50:2633–9. - PubMed
-
- Abramson S, Hoffstein ST, Weissmann G. Superoxide anion generation by human neutrophils exposed to monosodium urate. Arthritis Rheum. 1982;25:174–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

