Fetal alcohol exposure increases mammary tumor susceptibility and alters tumor phenotype in rats
- PMID: 20662802
- PMCID: PMC4634124
- DOI: 10.1111/j.1530-0277.2010.01276.x
Fetal alcohol exposure increases mammary tumor susceptibility and alters tumor phenotype in rats
Abstract
Background: Altered fetal programming because of a suboptimal in utero environment has been shown to increase susceptibility to many diseases later in life. This study examined the effect of alcohol exposure in utero on N-nitroso-N-methylurea (NMU)-induced mammary cancer risk during adulthood.
Methods: Study 1: Pregnant Sprague Dawley rats were fed a liquid diet containing 6.7% ethanol (alcohol-fed), an isocaloric liquid diet (pair-fed), or rat chow ad libitum (ad lib-fed) from day 11 to 21 of gestation. At birth, female pups were cross-fostered to ad lib-fed control dams. Adult offspring were given an I.P. injection of NMU at a dose of 50 mg/kg body weight. Mammary glands were palpated for tumors twice a week, and rats were euthanized at 23 weeks postinjection. Study 2: To investigate the role of estradiol (E2), animals were exposed to the same in utero treatments but were not given NMU. Serum was collected during the preovulatory phase of the estrous cycle.
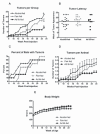
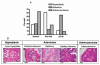
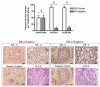
Results: At 16 weeks postinjection, overall tumor multiplicity was greater in the offspring from the alcohol-fed group compared to the control groups, indicating a decrease in tumor latency. At study termination, 70% of all animals possessed tumors. Alcohol-exposed animals developed more malignant tumors and more estrogen receptor-α-negative tumors relative to the control groups. In addition, IGF-binding protein-5 (IGFBP-5) mRNA and protein were decreased in tumors of alcohol-exposed animals. Study 2 showed that alcohol-fed animals had significantly increased circulating E2 when compared to either control group.
Conclusions: These data indicate that alcohol exposure in utero increases susceptibility to mammary tumorigenesis in adulthood and suggest that alterations in the IGF and E2 systems may play a role in the underlying mechanism.
Copyright © 2010 by the Research Society on Alcoholism.
Figures
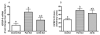
References
-
- American Cancer Society AC . Cancer Facts and Figures 2009. American Cancer Society; Atlanta: 2009.
-
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. - PubMed
-
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–2994. - PubMed
-
- Boutinaud M, Shand JH, Park MA, Phillips K, Beattie J, Flint DJ, Allan GJ. A quantitative RT-PCR study of the mRNA expression profile of the IGF axis during mammary gland development. J Mol Endocrinol. 2004;33:195–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

