Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial
- PMID: 20662805
- PMCID: PMC2965272
- DOI: 10.1111/j.1530-0277.2010.01273.x
Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial
Abstract
Background: Recent clinical trials and case-reports indicate that baclofen, a GABA(B) agonist, may have efficacy for alcohol dependence. Baclofen has been shown to enhance abstinence, to reduce drinking quantity, to reduce craving, and to reduce anxiety in alcohol-dependent individuals in 2 placebo-controlled trials in Italy. However, the clinical trial data with baclofen is limited. The purpose of the present study was to test the efficacy and tolerability of baclofen in alcohol dependence in the United States.
Methods: The study was a double-blind, placebo-controlled, randomized study comparing 30 mg/d of baclofen to placebo over 12 weeks of treatment and utilizing 8 sessions of BRENDA, a low-intensity psychosocial intervention. One hundred and twenty-one subjects were screened to yield 80 randomized subjects (44 men) with randomization balanced for gender. Percent heavy drinking days was the primary outcome measure with other drinking outcomes, anxiety levels, and craving as secondary outcomes. Tolerability was examined.
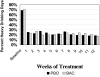
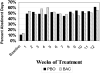
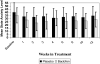
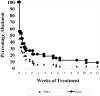
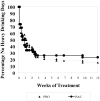
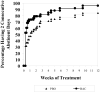
Results: Seventy-six percent of subjects completed the study. No difference by drug condition was seen in percentage of heavy drinking days where on-average rates were 25.5% (±23.6%) for placebo and 25.9% (±23.2%) for baclofen during treatment (t(73)=0.59, p=0.56). Similarly, no differences were seen by drug condition in percentage of days abstinent, time to first drink, or time to relapse to heavy drinking. Baclofen was associated with a significant reduction in state anxiety (F(1,73)= 5.39, p=0.02). Baclofen was well tolerated with only 2 individuals stopping baclofen because of adverse events. There were no serious adverse events.
Conclusions: Baclofen, a GABA(B) agonist, represents a possible new pharmacotherapeutic approach to alcohol dependence. Despite encouraging preclinical data and prior positive clinical trials with baclofen in Italy, the current trial did not find evidence that baclofen is superior to placebo in the treatment of alcohol dependence. Additional clinical trial work is necessary to establish whether baclofen does or does not have therapeutic efficacy in alcohol dependence and, if it does, what factors are predictive of response.
Copyright © 2010 by the Research Society on Alcoholism.
Figures
Comment in
-
High-dose baclofen for suppression of alcohol dependence.Alcohol Clin Exp Res. 2011 May;35(5):845-6; author reply 847. doi: 10.1111/j.1530-0277.2010.01412.x. Epub 2011 Feb 8. Alcohol Clin Exp Res. 2011. PMID: 21303382 No abstract available.
-
Baclofen effect related to liver damage.Alcohol Clin Exp Res. 2011 May;35(5):848. doi: 10.1111/j.1530-0277.2010.01414.x. Epub 2011 Feb 8. Alcohol Clin Exp Res. 2011. PMID: 21303383 No abstract available.
References
-
- Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med. 2006;119:276.e13–8. - PubMed
-
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II--Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. - PubMed
-
- Addolorato G, Leggio L, Abenavoli L, DeLorenzi G, Parente A, Caputo F, Janiri L, Capristo E, Rapaccini GL, Gasbarrini G. Suppression of alcohol delirium tremens by baclofen administration: a case report. Clin Neuropharmacol. 2003;26:258–262. - PubMed
-
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. - PubMed
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D’Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

