Role of microglia in ethanol's apoptotic action on hypothalamic neuronal cells in primary cultures
- PMID: 20662807
- PMCID: PMC2965273
- DOI: 10.1111/j.1530-0277.2010.01271.x
Role of microglia in ethanol's apoptotic action on hypothalamic neuronal cells in primary cultures
Abstract
Background: Microglia are the major inflammatory cells in the central nervous system and play a role in brain injuries as well as brain diseases. In this study, we determined the role of microglia in ethanol's apoptotic action on neuronal cells obtained from the mediobasal hypothalamus and maintained in primary cultures. We also tested the effect of cAMP, a signaling molecule critically involved in hypothalamic neuronal survival, on microglia-mediated ethanol's neurotoxic action.
Methods: Ethanol's neurotoxic action was determined on enriched fetal mediobasal hypothalamic neuronal cells with or without microglia cells or ethanol-activated microglia-conditioned media. Ethanol's apoptotic action was determined using nucleosome assay. Microglia activation was determined using OX6 histochemistry and by measuring inflammatory cytokines secretion from microglia in cultures using enzyme-linked immunosorbent assay (ELISA). An immunoneutralization study was conducted to identify the role of a cytokine involved in ethanol's apoptotic action.
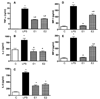
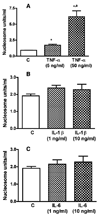
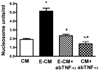
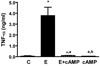
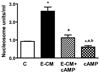
Results: We show here that ethanol at a dose range of 50 and 100 mM induces neuronal death by an apoptotic process. Ethanol's ability to induce an apoptotic death of neurons is increased by the presence of ethanol-activated microglia-conditioned media. In the presence of ethanol, microglia showed elevated secretion of various inflammatory cytokines, of which TNF-α shows significant apoptotic action on mediobasal hypothalamic neuronal cells. Ethanol's neurotoxic action was completely prevented by cAMP. The cell-signaling molecule also prevented ethanol-activated microglial production of TNF-α. Immunoneutralization of TNF-α prevented the microglia-derived media's ability to induce neuronal death.
Conclusions: These results suggest that ethanol's apoptotic action on hypothalamic neuronal cells might be mediated via microglia, possibly via increased production of TNF-α. Furthermore, cAMP reduces TNF-α production from microglia to prevent ethanol's neurotoxic action.
Copyright © 2010 by the Research Society on Alcoholism.
Figures


Similar articles
-
Mu-opioid receptor and delta-opioid receptor differentially regulate microglial inflammatory response to control proopiomelanocortin neuronal apoptosis in the hypothalamus: effects of neonatal alcohol.J Neuroinflammation. 2017 Apr 14;14(1):83. doi: 10.1186/s12974-017-0844-3. J Neuroinflammation. 2017. PMID: 28407740 Free PMC article.
-
Cyclic adenosine monophosphate and brain-derived neurotrophic factor decreased oxidative stress and apoptosis in developing hypothalamic neuronal cells: role of microglia.Alcohol Clin Exp Res. 2013 Aug;37(8):1370-9. doi: 10.1111/acer.12104. Epub 2013 Mar 29. Alcohol Clin Exp Res. 2013. PMID: 23550806 Free PMC article.
-
Ethanol-activated microglial exosomes induce MCP1 signaling mediated death of stress-regulatory proopiomelanocortin neurons in the developing hypothalamus.J Neuroinflammation. 2024 Oct 30;21(1):279. doi: 10.1186/s12974-024-03274-6. J Neuroinflammation. 2024. PMID: 39478585 Free PMC article.
-
Exposure to low and moderate doses of alcohol on late gestation modifies infantile response to and preference for alcohol in rats.Ann Ist Super Sanita. 2006;42(1):22-30. Ann Ist Super Sanita. 2006. PMID: 16801722 Review.
-
Heterogeneity of microglia and TNF signaling as determinants for neuronal death or survival.Neurotoxicology. 2009 Sep;30(5):785-93. doi: 10.1016/j.neuro.2009.07.001. Epub 2009 Jul 9. Neurotoxicology. 2009. PMID: 19596372 Free PMC article. Review.
Cited by
-
Neuroimmune mechanisms in fetal alcohol spectrum disorder.Dev Neurobiol. 2012 Oct;72(10):1302-16. doi: 10.1002/dneu.22035. Epub 2012 Sep 1. Dev Neurobiol. 2012. PMID: 22623427 Free PMC article. Review.
-
Alcohol Increases Exosome Release from Microglia to Promote Complement C1q-Induced Cellular Death of Proopiomelanocortin Neurons in the Hypothalamus in a Rat Model of Fetal Alcohol Spectrum Disorders.J Neurosci. 2020 Oct 7;40(41):7965-7979. doi: 10.1523/JNEUROSCI.0284-20.2020. Epub 2020 Sep 4. J Neurosci. 2020. PMID: 32887744 Free PMC article.
-
Effects of alcohol on the endocrine system.Endocrinol Metab Clin North Am. 2013 Sep;42(3):593-615. doi: 10.1016/j.ecl.2013.05.008. Endocrinol Metab Clin North Am. 2013. PMID: 24011889 Free PMC article. Review.
-
Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2.Alcohol Clin Exp Res. 2017 May;41(5):939-954. doi: 10.1111/acer.13368. Epub 2017 Mar 30. Alcohol Clin Exp Res. 2017. PMID: 28273337 Free PMC article.
-
Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective.Front Integr Neurosci. 2016 Jan 11;9:65. doi: 10.3389/fnint.2015.00065. eCollection 2015. Front Integr Neurosci. 2016. PMID: 26793073 Free PMC article. Review.
References
-
- Allen RT, Hunter WJ, III, Agrawal WJDK. Morphological and biological characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997;37:215–228. 3rd. - PubMed
-
- Boyadjieva N, Sarkar DK. Effects of ethanol on basal and prostaglandin E1-induced increases in beta-endorphin release and intracellular cAMP levels in hypothalamic cells. Alcoholism: Clin Exp Res. 1997;21:1005–1009. - PubMed
-
- Boyadjieva N, Sarkar DK. Effects of ethanol on basal and adenosine-induced increases in beta-endorphin release and intracellular cAMP levels in hypothalamic cells. Brain Res. 1999;824:112–118. - PubMed
-
- Brown RE. An Introduction to Neuroendocrinology. New York, NY: Cambridge University Press; 1998. pp. 41–43.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

