Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain
- PMID: 20663017
- PMCID: PMC2939954
- DOI: 10.1111/j.1471-4159.2010.06899.x
Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain
Abstract
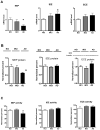
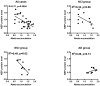
The brain steady state level of β-amyloid (Aβ) is determined by the balance between its production and removal, the latter through egress across blood and CSF barriers as well as Aβ degradation. The major Aβ-degrading enzymes are neprilysin (NEP), insulin-degrading enzyme (IDE), and endothelin-converting enzyme (ECE-1). Although evidence suggests that NEP is down-regulated in Alzheimer's disease (AD), the role of IDE and ECE in the Aβ accumulation in aging and dementia remains less certain. In this study, we examined mRNA and protein expression, as well as biological activity of NEP, IDE, and ECE-1 in human frontal cortex by real-time RT-PCR for mRNA, immunoblotting for protein, and highly sensitive and specific fluorescence assays for activity. The relationships between Aβ-degrading enzymes and pathologic measures and clinical features were also assessed. The results showed that NEP mRNA, protein level, and activity were decreased in AD compared with normal controls with no cognitive impairment (NCI). In contrast, IDE activity was unchanged, but there was higher expression of IDE mRNA, indicating a possible compensatory reaction because of deficits in activity. ECE-1 expression in AD brain showed no significant difference compared with age-matched controls. Correlation analyses suggested that NEP expression was correlated with Aβ accumulation and clinical diagnosis, being lower in AD than in no cognitive impairment. In contrast, neither IDE nor ECE-1 correlated with Aβ or clinical diagnosis. These findings provide additional support for NEP as the major protease involved in Aβ degradation and suggest its possible therapeutic targeting in AD.
© 2010 The Authors. Journal Compilation © 2010 International Society for Neurochemistry.
Figures
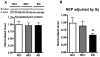


Similar articles
-
Impaired amyloid β-degrading enzymes in brain of streptozotocin-induced diabetic rats.J Endocrinol Invest. 2011 Jan;34(1):26-31. doi: 10.1007/BF03346691. Epub 2010 Apr 22. J Endocrinol Invest. 2011. PMID: 20414044
-
Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer's disease.J Alzheimers Dis. 2015;44(4):1291-302. doi: 10.3233/JAD-142463. J Alzheimers Dis. 2015. PMID: 25408216
-
Effects of 4-hydroxy-nonenal and Amyloid-beta on expression and activity of endothelin converting enzyme and insulin degrading enzyme in SH-SY5Y cells.J Alzheimers Dis. 2009;17(3):489-501. doi: 10.3233/JAD-2009-1066. J Alzheimers Dis. 2009. PMID: 19363254 Free PMC article.
-
Impact of Insulin Degrading Enzyme and Neprilysin in Alzheimer's Disease Biology: Characterization of Putative Cognates for Therapeutic Applications.J Alzheimers Dis. 2015;48(4):891-917. doi: 10.3233/JAD-150379. J Alzheimers Dis. 2015. PMID: 26444774 Review.
-
Molecular basis of selective amyloid-β degrading enzymes in Alzheimer's disease.FEBS J. 2024 Jul;291(14):2999-3029. doi: 10.1111/febs.16939. Epub 2023 Sep 8. FEBS J. 2024. PMID: 37622248 Review.
Cited by
-
Drosophila Neprilysins Are Involved in Middle-Term and Long-Term Memory.J Neurosci. 2016 Sep 14;36(37):9535-46. doi: 10.1523/JNEUROSCI.3730-15.2016. J Neurosci. 2016. PMID: 27629706 Free PMC article.
-
The roles of microRNAs in spinal cord ischemia-reperfusion injury.Neural Regen Res. 2022 Dec;17(12):2593-2599. doi: 10.4103/1673-5374.339471. Neural Regen Res. 2022. PMID: 35662187 Free PMC article. Review.
-
Regulatory feedback cycle of the insulin-degrading enzyme and the amyloid precursor protein intracellular domain: Implications for Alzheimer's disease.Aging Cell. 2020 Nov;19(11):e13264. doi: 10.1111/acel.13264. Epub 2020 Oct 31. Aging Cell. 2020. PMID: 33128835 Free PMC article.
-
Target Enzymes Considered for the Treatment of Alzheimer's Disease and Parkinson's Disease.Biomed Res Int. 2020 Nov 9;2020:2010728. doi: 10.1155/2020/2010728. eCollection 2020. Biomed Res Int. 2020. PMID: 33224974 Free PMC article. Review.
-
Gender, sex steroid hormones, and Alzheimer's disease.Horm Behav. 2013 Feb;63(2):301-7. doi: 10.1016/j.yhbeh.2012.04.006. Epub 2012 Apr 19. Horm Behav. 2013. PMID: 22554955 Free PMC article. Review.
References
-
- Ahn K, Herman SB, Fahnoe DC. Soluble human endothelin-converting enzyme-1: expression, purification, and demonstration of pronounced pH sensitivity. Arch Biochem Biophys. 1998;359:258–268. - PubMed
-
- Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL. Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid beta-protein (Abeta) deposition. Brain Res. 2001;902:277–281. - PubMed
-
- Apelt J, Ach K, Schliebs R. Aging-related down-regulation of neprilysin, a putative beta-amyloid-degrading enzyme, in transgenic Tg2576 Alzheimer-like mouse brain is accompanied by an astroglial upregulation in the vicinity of beta-amyloid plaques. Neurosci Lett. 2003;339:183–186. - PubMed
-
- Barnes K, Turner AJ. The endothelin system and endothelin-converting enzyme in the brain: molecular and cellular studies. Neurochem Res. 1997;22:1033–1040. - PubMed
-
- Bates KA, Verdile G, Li QX, Ames D, Hudson P, Masters CL, Martins RN. Clearance mechanisms of Alzheimer’s amyloid-beta peptide: implications for therapeutic design and diagnostic tests. Mol Psychiatry. 2009;14:469–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

