Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity
- PMID: 20663192
- PMCID: PMC2918604
- DOI: 10.1186/1476-9255-7-37
Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity
Abstract
Background: Dendritic cells (DC) are main gate-keepers of the immune system, bridging the innate and adaptive immune system. DCs are able to mature into inflammatory DCs at sites of inflammation in both autoimmune and allergic disease, thereby sustaining a continuous activation of the adaptive immune system at sites of inflammation. This function of DCs makes them attractive target cells for therapeutic intervention in inflammatory diseases. We have designed a DC-based screening model by which drug candidates can be evaluated for their ability to suppress DC maturation into an inflammatory and disease promoting phenotype.
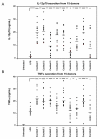
Methods: Human monocyte derived DCs were differentiated using IL-4 and GM-CSF to immature DCs (imDCs). The imDCs were treated with various combinations of TLR-agonists and pro-inflammatory cytokines to identify cocktails with ability to mature imDCs into inflammatory DCs. The effect of the cocktails on DC maturation was evaluated using ELISA and cytokine arrays to measure secreted cytokines and chemokines. FACS analysis was used to assess expression of maturation markers, and functional studies were carried out using naïve allogeneic T-cells to assay for a Th1-promoting DC phenotype.
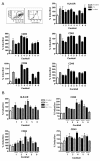
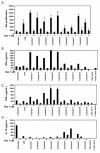
Results: Nine cocktails were designed with potent ability to induce secretion of the Th1-promoting cytokines IL-12p70 and TNFalpha from imDCs, and three were able to induce the Th17-promoting cytokine IL-23. The cocktails were further characterized using cytokine arrays, showing induction of inflammation related cytokines and chemokines like CXCL10, CCL2, CCL4, CCL8, CCL15, CCL20 and IL-8, of which some are present in a range of autoimmune pathologies. Prostaglandin E2 secretion was identified from DCs treated with TLR agonists poly I:C and peptidoglycan, but not LPS. The cocktails were able to induce DC maturation markers like HLA-DR, CD40, CD80, CD83 and CD86, except the TLR7/8 agonist R848. Functional end-points made by co-culture of allogeneic CD4+ T cells with the cocktail treated DCs, showed that five cocktails in particular could induce a classical Th1-phenotype with ability to secrete high amounts of the hall-mark cytokine IFNgamma. The model was validated using dexamethasone and two COX-inhibitors, which were able to suppress the cocktail driven pro-inflammatory DC maturation.
Conclusions: The identification of novel Th1-promoting cocktails allows screening of anti-inflammatory drug candidates by assessing the ability to suppress the activation and differentiation of imDCs into inflammatory DCs with a specific Th1-promoting phenotype. The model thus provides a screening tool, which can identify potential anti-inflammatory effects on the natural regulator of the immune response, the dendritic cell.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

