TRAIL sensitize MDR cells to MDR-related drugs by down-regulation of P-glycoprotein through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases
- PMID: 20663232
- PMCID: PMC2918570
- DOI: 10.1186/1476-4598-9-199
TRAIL sensitize MDR cells to MDR-related drugs by down-regulation of P-glycoprotein through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases
Abstract
Background: The development of new modulator possessing high efficacy, low toxicity and high selectivity is a pivotal approach to overcome P-glycoprotein (P-gp) mediated multidrug resistance (MDR) in cancer treatment. In this study, we suggest a new molecular mechanism that TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) down-regulates P-glycoprotein (P-gp) through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases and thereby sensitize MDR cells to MDR-related drugs.
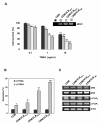
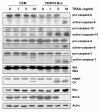
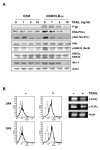
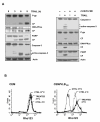
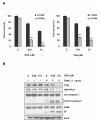
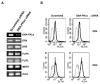
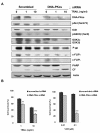
Results: MDR variants, CEM/VLB10-2, CEM/VLB55-8 and CEM/VLB100 cells, with gradually increased levels of P-gp derived from human lymphoblastic leukemia CEM cells, were gradually more susceptible to TRAIL-induced apoptosis and cytotoxicity than parental CEM cells. The P-gp level of MDR variants was positively correlated with the levels of DNA-PKcs, pAkt, pGSK-3beta and c-Myc as well as DR5 and negatively correlated with the level of c-FLIPs. Hypersensitivity of CEM/VLB100 cells to TRAIL was accompanied by the activation of mitochondrial apoptotic pathway as well as the activation of initiator caspases. In addition, TRAIL-induced down-regulation of DNA-PKcs/Akt/GSK-3beta pathway and c-FLIP and up-regulation of cell surface expression of death receptors were associated with the increased susceptibility to TRAIL of MDR cells. Moreover, TRAIL inhibited P-gp efflux function via caspase-3-dependent degradation of P-gp as well as DNA-PKcs and subsequently sensitized MDR cells to MDR-related drugs such as vinblastine and doxorubicin. We also found that suppression of DNA-PKcs by siRNA enhanced the susceptibility of MDR cells to vincristine as well as TRAIL via down-regulation of c-FLIP and P-gp expression and up-regulation of DR5.
Conclusion: This study showed for the first time that the MDR variant of CEM cells was hypersensitive to TRAIL due to up-regulation of DR5 and concomitant down-regulation of c-FLIP, and degradation of P-gp and DNA-PKcs by activation of caspase-3 might be important determinants of TRAIL-induced sensitization of MDR cells to MDR-related drugs. Therefore, combination of TRAIL and chemotherapeutic drugs may be a good strategy for treatment of cancer with multidrug resistance.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

