Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3
- PMID: 20663464
- PMCID: PMC2909532
- DOI: 10.1177/193229681000400429
Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3
Abstract
Background: Continuous subcutaneous insulin infusion (CSII) by means of insulin pump devices is considered to be one of the most optimal therapies to achieve treatment targets in patients with diabetes mellitus. In CSII, the insulin is delivered through Teflon catheters or steel needle infusion sets, which need to be renewed on a regular basis. This pilot study was performed to investigate the optimal change frequency in daily practice and to explore potential problems that may occur when the sets are used for a more prolonged time than the recommended up to 72 hours of usage (Teflon catheters).
Method: Twelve patients with type 1 diabetes participated in the trial [age (mean +/- STD): 40.3 +/- 12.6 years, body mass index: 26.2 +/- 3.3 kg/m(2), hemoglobin A1c: 6.7 +/- 0.6%]. They were asked to wear their infusion set (Comfort or Silhouette) for increasing periods of 1, 2, 3, 4, and 5 days. After each use, patients completed standardized questionnaires regarding technical and medical issues associated with infusion set use. A health care professional investigated the infusion sites and infusion sets and completed an "infusion set inspection" questionnaire. Blood glucose was measured and recorded to assess a potential influence of duration of catheter use on glycemic control.
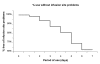
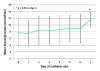
Results: Infusion set and injection site problems (itching, bruising, swelling, and pain) started to occur in measurable amounts on the 3rd day of catheter use, and about 40% of patients reported significant issues when using a catheter for 5 days. In parallel, there was a consistent increase in mean daily blood glucose levels that correlated with the number of days of catheter use (e.g., day 1: 7.5 +/- 3.8 mmol/liter, day 3: 8.4 +/- 4.2 mmol/liter, day 5: 9.0 +/- 4.0 mmol/liter, day 7: 11.6 +/- 2.2 mmol/liter, p < 0.05 vs day 1).
Conclusions: Using the catheters for 2 days resulted in a safe and well-tolerated therapy. Clinically relevant adverse events started to occur during the 3rd day and their incidence increased constantly with longer use. This was associated with undesired changes in mean glycemic control. Data support the recommendation by the drug and device manufacturers that insulin pump catheters should only be used for 48-72 hours to avoid adverse events and potential metabolic deterioration.
2010 Diabetes Technology Society.
Figures


References
-
- Pfützner A, Berger S, Spinas GA. Aktueller stellenwert der kontinuierlichen subkutanen Insulininfusion (CSII) mit Insulinpumpen in der therapie des diabetes mellitus (Actual value of CSII in the therapy of diabetes mellitus) Swiss Med Weekly. 2000;130:1954–1961. - PubMed
-
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26(4):1079–1087. - PubMed
-
- Pickup JC, Renard E. Long-acting insulin analogs versus insulin pump therapy for the treatment of type 1 and type 2 diabetes. Diabetes Care. 2008;31(Suppl. 2):S140–S145. - PubMed
-
- Berthe E, Lireux B, Coffin C, Goulet-Salmon B, Houlbert D, Boutreux S, Fradin S, Reznik Y. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res. 2007;39(3):224–229. - PubMed
-
- Scheiner G, Sobel RJ, Smith DE, Pick AJ, Kruger D, King J, Green K. Insulin pump therapy: guidelines for successful outcomes. Diabetes Educ. 2009;35(Suppl. 2):29S, 41S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

