Comparison of MRI properties between derivatized DTPA and DOTA gadolinium-dendrimer conjugates
- PMID: 20663676
- PMCID: PMC2918719
- DOI: 10.1016/j.bmc.2010.06.086
Comparison of MRI properties between derivatized DTPA and DOTA gadolinium-dendrimer conjugates
Abstract
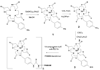
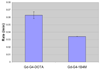
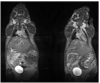
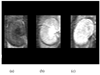
In this report we directly compare the in vivo and in vitro MRI properties of gadolinium-dendrimer conjugates of derivatized acyclic diethylenetriamine-N,N',N',N'',N''-pentaacetic acid (1B4M-DTPA) and macrocyclic 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid (C-DOTA). The metal-ligand chelates were pre-formed in alcohol prior to conjugation to the generation 4 PAMAM dendrimer (G4D), and the dendrimer-based agents were purified by Sephadex(R) G-25 column. The analysis and SE-HPLC data indicated chelate to dendrimer ratios of 30:1 and 28:1, respectively. Molar relaxivity measured at pH 7.4, 22 degrees C, and 3T are comparable (29.5 vs 26.9 mM(-1)s(-1)), and both conjugates are equally viable as MRI contrast agents based on the images obtained. The macrocyclic agent however exhibits a faster rate of clearance in vivo (t(1/2)=16 vs 29 min). Our conclusion is that the macrocyclic-based agent is the more suitable agent for in vivo use for these reasons combined with kinetic inertness associated with the Gd(III) DOTA complex stability properties.
Published by Elsevier Ltd.
Figures


Similar articles
-
Preparation, characterization and in vivo assessment of Gd-albumin and Gd-dendrimer conjugates as intravascular contrast-enhancing agents for MRI.J Inorg Biochem. 2011 May;105(5):722-7. doi: 10.1016/j.jinorgbio.2011.01.017. Epub 2011 Apr 2. J Inorg Biochem. 2011. PMID: 21463567 Free PMC article.
-
Poly(amidoamine) dendrimer based MRI contrast agents exhibiting enhanced relaxivities derived via metal preligation techniques.Bioconjug Chem. 2010 Jun 16;21(6):1014-7. doi: 10.1021/bc1000802. Bioconjug Chem. 2010. PMID: 20462240 Free PMC article.
-
A new approach in the preparation of dendrimer-based bifunctional diethylenetriaminepentaacetic acid MR contrast agent derivatives.Bioconjug Chem. 2009 Jul;20(7):1412-8. doi: 10.1021/bc900057z. Bioconjug Chem. 2009. PMID: 19555072 Free PMC article.
-
Cyclen-based Gd3+ complexes as MRI contrast agents: Relaxivity enhancement and ligand design.Bioorg Med Chem. 2016 Nov 15;24(22):5663-5684. doi: 10.1016/j.bmc.2016.09.069. Epub 2016 Oct 1. Bioorg Med Chem. 2016. PMID: 27729196 Review.
-
Gadolinium-1,4,7,10-tetraazacyclododecane-N,N’,N’,N’”-tetraacetic-monoamide-24-cascade-polymer.2007 Oct 17 [updated 2007 Dec 4]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Oct 17 [updated 2007 Dec 4]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641779 Free Books & Documents. Review.
Cited by
-
Biodegradable polydisulfide dendrimer nanoclusters as MRI contrast agents.ACS Nano. 2012 Nov 27;6(11):9416-24. doi: 10.1021/nn304160p. Epub 2012 Oct 29. ACS Nano. 2012. PMID: 23098069 Free PMC article.
-
[18F]CB251 PET/MR imaging probe targeting translocator protein (TSPO) independent of its Polymorphism in a Neuroinflammation Model.Theranostics. 2020 Jul 23;10(20):9315-9331. doi: 10.7150/thno.46875. eCollection 2020. Theranostics. 2020. PMID: 32802194 Free PMC article.
-
A Manganese Porphyrin Platform for the Design and Synthesis of Molecular and Targeted MRI Contrast Agents.Int J Mol Sci. 2023 May 31;24(11):9532. doi: 10.3390/ijms24119532. Int J Mol Sci. 2023. PMID: 37298480 Free PMC article.
-
Preparation of cystamine core dendrimer and antibody-dendrimer conjugates for MRI angiography.Mol Pharm. 2012 Mar 5;9(3):374-81. doi: 10.1021/mp2003219. Epub 2011 Sep 21. Mol Pharm. 2012. PMID: 21882823 Free PMC article.
-
Gd-based macromolecules and nanoparticles as magnetic resonance contrast agents for molecular imaging.Curr Top Med Chem. 2013;13(4):411-21. doi: 10.2174/1568026611313040002. Curr Top Med Chem. 2013. PMID: 23432004 Free PMC article. Review.
References
-
- Caravan P. Chem. Soc. Rev. 2006;35:512. - PubMed
-
- Wang SJ, Brechbiel M, Wiener EC. Invest. Radiol. 2003;38:662. - PubMed
-
- Kobayashi H, Brechbiel MW. Mol. Imaging. 2003;2:1. - PubMed
-
- Sato N, Kobayashi H, Hiraga A, Saga T, Togashi K, Konishi J, Brechbiel MW. Magn. Reson. Med. 2001;46:1169. - PubMed
-
- Tomalia DA, Reyna LA, Svenson S. Biochem. Soc. Trans. 2007;35:61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

