Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration
- PMID: 20663882
- PMCID: PMC2945578
- DOI: 10.1074/jbc.M110.110957
Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration
Abstract
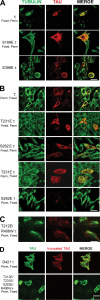
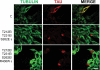
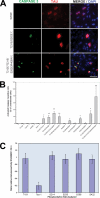
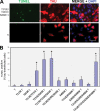
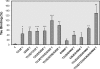
Abnormal hyperphosphorylation of the microtubule-associated protein Tau is a hallmark of Alzheimer disease and related diseases called tauopathies. As yet, the exact mechanism by which this pathology causes neurodegeneration is not understood. The present study provides direct evidence that Tau abnormal hyperphosphorylation causes its aggregation, breakdown of the microtubule network, and cell death and identifies phosphorylation sites involved in neurotoxicity. We generated pseudophosphorylated Tau proteins by mutating Ser/Thr to Glu and, as controls, to Ala. These mutations involved one, two, or three pathological phosphorylation sites by site-directed mutagenesis using as backbones the wild type or FTDP-17 mutant R406W Tau. Pseudophosphorylated and corresponding control Tau proteins were expressed transiently in PC12 and CHO cells. We found that a single phosphorylation site alone had little influence on the biological activity of Tau, except Thr(212), which, upon mutation to Glu in the R406W background, induced Tau aggregation in cells, suggesting phosphorylation at this site along with a modification on the C-terminal of the protein facilitates self-assembly of Tau. The expression of R406W Tau pseudophosphorylated at Thr(212), Thr(231), and Ser(262) triggered caspase-3 activation in as much as 85% of the transfected cells, whereas the corresponding value for wild type pseudophosphorylated Tau was 30%. Cells transfected with pseudophosphorylated Tau became TUNEL-positive.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

