Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes
- PMID: 20663895
- PMCID: PMC2943265
- DOI: 10.1074/jbc.M110.139493
Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes
Abstract
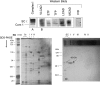
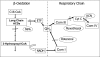
Fatty acid β-oxidation (FAO) and oxidative phosphorylation (OXPHOS) are key pathways involved in cellular energetics. Reducing equivalents from FAO enter OXPHOS at the level of complexes I and III. Genetic disorders of FAO and OXPHOS are among the most frequent inborn errors of metabolism. Patients with deficiencies of either FAO or OXPHOS often show clinical and/or biochemical findings indicative of a disorder of the other pathway. In this study, the physical and functional interactions between these pathways were examined. Extracts of isolated rat liver mitochondria were subjected to blue native polyacrylamide gel electrophoresis (BNGE) to separate OXPHOS complexes and supercomplexes followed by Western blotting using antisera to various FAO enzymes. Extracts were also subjected to sucrose density centrifugation and fractions analyzed by BNGE or enzymatic assays. Several FAO enzymes co-migrated with OXPHOS supercomplexes in different patterns in the gels. When palmitoyl-CoA was added to the sucrose gradient fractions containing OXPHOS supercomplexes in the presence of potassium cyanide, cytochrome c was reduced. Cytochrome c reduction was completely blocked by myxothiazol (a complex III inhibitor) and 3-mercaptopropionate (an inhibitor of the first step of FAO), but was only partially inhibited by rotenone (a complex I inhibitor). Although palmitoyl-CoA and octanoyl-CoA provided reducing equivalents to OXPHOS-containing supercomplex fractions, no accumulation of their intermediates was detected. In contrast, short branched acyl-CoA substrates were not metabolized by OXPHOS-containing supercomplex fractions. These data provide evidence of a multifunctional FAO complex within mitochondria that is physically associated with OXPHOS supercomplexes and promotes metabolic channeling.
Figures






References
-
- Cruciat C. M., Brunner S., Baumann F., Neupert W., Stuart R. A. (2000) J. Biol. Chem. 275, 18093–18098 - PubMed
-
- Schägger H. (2001) IUBMB Life 52, 119–128 - PubMed
-
- Eubel H., Heinemeyer J., Sunderhaus S., Braun H. P. (2004) Plant Physiol. Biochem. 42, 937–942 - PubMed
-
- Krause F., Reifschneider N. H., Goto S., Dencher N. A. (2005) Biochem. Biophys. Res. Commun. 329, 583–590 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

