A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter
- PMID: 20663903
- PMCID: PMC2922429
- DOI: 10.1158/0008-5472.CAN-10-0686
A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter
Abstract
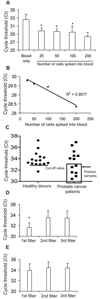
Circulating tumor cells (CTC) quantified in cancer patients' blood can predict disease outcome and response to therapy. However, the CTC analysis platforms commonly used cannot capture live CTCs and only apply to tumors of epithelial origin. To address these limitations, we have developed a novel cancer detection platform which measures telomerase activity from live CTCs captured on a parylene-C slot microfilter. Using a constant low-pressure delivery system, the new microfilter platform was capable of cell capture from 1 mL of whole blood in less than 5 minutes, achieving 90% capture efficiency, 90% cell viability, and 200-fold sample enrichment. Importantly, the captured cells retained normal morphology by scanning electron microscopy and could be readily manipulated, further analyzed, or expanded on- or off-filter. Telomerase activity--a well-recognized universal cancer marker--was reliably detected by quantitative PCR from as few as 25 cancer cells added into 7.5 mL of whole blood and captured on the microfilter. Moreover, significant telomerase activity elevation was also measured from patients' blood samples and from single cancer cells lifted off of the microfilter. Live CTC capture and analysis is fast and simple yet highly quantitative, versatile, and applicable to nearly all solid tumor types, making this a highly promising new strategy for cancer detection and characterization.
(c)2010 AACR.
Conflict of interest statement
Figures



Similar articles
-
Circulating tumor cell telomerase activity as a prognostic marker for overall survival in SWOG 0421: a phase III metastatic castration resistant prostate cancer trial.Int J Cancer. 2015 Apr 15;136(8):1856-62. doi: 10.1002/ijc.29212. Epub 2014 Oct 8. Int J Cancer. 2015. PMID: 25219358 Free PMC article. Clinical Trial.
-
Microfilter-Based Capture and Release of Viable Circulating Tumor Cells.Methods Mol Biol. 2017;1634:93-105. doi: 10.1007/978-1-4939-7144-2_7. Methods Mol Biol. 2017. PMID: 28819843
-
Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells.J Chromatogr A. 2007 Aug 31;1162(2):154-61. doi: 10.1016/j.chroma.2007.05.064. Epub 2007 May 29. J Chromatogr A. 2007. PMID: 17561026
-
Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells.Acc Chem Res. 2014 Oct 21;47(10):2941-50. doi: 10.1021/ar5001617. Epub 2014 Aug 11. Acc Chem Res. 2014. PMID: 25111636 Free PMC article. Review.
-
[Recent advances in isolation and detection of circulating tumor cells with a microfluidic system].Se Pu. 2022 Mar 8;40(3):213-223. doi: 10.3724/SP.J.1123.2021.07009. Se Pu. 2022. PMID: 35243831 Free PMC article. Review. Chinese.
Cited by
-
A cell transportation solution that preserves live circulating tumor cells in patient blood samples.BMC Cancer. 2016 May 6;16:300. doi: 10.1186/s12885-016-2330-1. BMC Cancer. 2016. PMID: 27150191 Free PMC article.
-
Immunomagnetic Isolation of HER2-Positive Breast Cancer Cells Using a Microfluidic Device.ACS Omega. 2023 Jun 6;8(24):21745-21754. doi: 10.1021/acsomega.3c01287. eCollection 2023 Jun 20. ACS Omega. 2023. PMID: 37360498 Free PMC article.
-
Circulating tumor cell isolation: the assets of filtration methods with polycarbonate track-etched filters.Chin J Cancer Res. 2015 Oct;27(5):479-87. doi: 10.3978/j.issn.1000-9604.2015.09.01. Chin J Cancer Res. 2015. PMID: 26543334 Free PMC article. Review.
-
Circulating tumor cells in prostate cancer diagnosis and monitoring: an appraisal of clinical potential.Mol Diagn Ther. 2014 Aug;18(4):389-402. doi: 10.1007/s40291-014-0101-8. Mol Diagn Ther. 2014. PMID: 24809501 Free PMC article.
-
Capture and Release of Viable Circulating Tumor Cells from Blood.J Vis Exp. 2016 Oct 28;(116):54435. doi: 10.3791/54435. J Vis Exp. 2016. PMID: 27842345 Free PMC article.
References
-
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. - PubMed
-
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. - PubMed
-
- Gasent Blesa JM, Alberola Candel V, Esteban Gonzalez E, et al. Circulating tumor cells in breast cancer: methodology and clinical repercussions. Clin Transl Oncol. 2008;10:399–406. - PubMed
-
- Goodman OB, Jr, Fink LM, Symanowski JT, et al. Circulating tumor cells in patients with castration-resistant prostate cancer baseline values and correlation with prognostic factors. Cancer Epidemiol Biomarkers Prev. 2009;18:1904–1913. - PubMed
-
- Ignatiadis M, Xenidis N, Perraki M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007;25:5194–5202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

