Vascular endothelial growth factor is a promising therapeutic target for the treatment of clear cell carcinoma of the ovary
- PMID: 20663925
- PMCID: PMC2941981
- DOI: 10.1158/1535-7163.MCT-10-0169
Vascular endothelial growth factor is a promising therapeutic target for the treatment of clear cell carcinoma of the ovary
Abstract
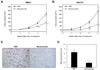
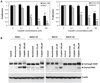
This study examines the role of vascular endothelial growth factor (VEGF) as a therapeutic target in clear cell carcinoma (CCC) of the ovary, which has been regarded as a chemoresistant histologic subtype. Immunohistochemical analysis using tissue microarrays of 98 primary ovarian cancers revealed that VEGF was strongly expressed both in early-stage and advanced-stage CCC of the ovary. In early-stage CCCs, patients who had tumors with high levels of VEGF had significantly shorter survival than those with low levels of VEGF. In vitro experiments revealed that VEGF expression was significantly higher in cisplatin-refractory human CCC cells (RMG1-CR and KOC7C-CR), compared with the respective parental cells (RMG1 and KOC7C) in the presence of cisplatin. In vivo treatment with bevacizumab markedly inhibited the growth of both parental CCC cell-derived (RMG1 and KOC7C) and cisplatin-refractory CCC cell-derived (RMG1-CR and KOC7C-CR) tumors as a result of inhibition of tumor angiogenesis. The results of the current study indicate that VEGF is frequently expressed and can be a promising therapeutic target in the management of CCC. Bevacizumab may be efficacious not only as a first-line treatment but also as a second-line treatment of recurrent disease in patients previously treated with cisplatin.
(c) 2010 AACR.
Conflict of interest statement
Figures







References
-
- American Cancer Society.: Cancer Facts and Figures 2009. Atlanta, Ga: http://www.cancer.org/downloads/STT/500809web.pdf.
-
- Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. - PubMed
-
- Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34:S1–S15. - PubMed
-
- Serov SF, Scully RE, Sobin LH. Histologic Typing of Ovarian Tumors. Vol. 9. Geneva: World Health Organization; 1973. International histologic classification of tumors.
-
- Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

