Efficient gene transfer into the mouse lung by fetal intratracheal injection of rAAV2/6.2
- PMID: 20664525
- PMCID: PMC2997580
- DOI: 10.1038/mt.2010.153
Efficient gene transfer into the mouse lung by fetal intratracheal injection of rAAV2/6.2
Abstract
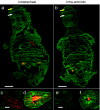
Fetal gene therapy is one of the possible new therapeutic strategies for congenital or perinatal diseases with high mortality or morbidity. We developed a novel delivery strategy to inject directly into the fetal mouse trachea. Intratracheal (i.t.) injection at embryonic day 18 (E18) was more efficient in targeting the fetal lung than conventional intra-amniotic (i.a.) delivery. Viral vectors derived from adeno-associated virus serotype 6.2, with tropism for the airway epithelium and not earlier tested in the fetal mouse lung, were injected into the fetal trachea. Bioluminescence (BL) imaging (BLI) was combined with magnetic resonance (MR) imaging (MRI) for noninvasive and accurate localization of transgene expression in vivo. Histological analysis for β-galactosidase (β-gal) revealed 17.5% of epithelial cells transduced in the conducting airways and 1.5% in the alveolar cells. Stable gene expression was observed up to 1 month after injection. This study demonstrates that direct injection of rAAV2/6.2 in the fetal mouse trachea is superior to i.a. delivery for transducing the lung. Second, as stable gene transfer was detected up to 1 postnatal month, this approach may be useful to evaluate fetal gene therapy for pulmonary diseases such as cystic fibrosis, requiring both substantial numbers of transduced cells as well as prolonged gene expression to obtain a stable phenotypic effect.
Figures




Similar articles
-
Noninvasive Imaging Reveals Stable Transgene Expression in Mouse Airways After Delivery of a Nonintegrating Recombinant Adeno-Associated Viral Vector.Hum Gene Ther. 2016 Jan;27(1):60-71. doi: 10.1089/hum.2015.109. Epub 2015 Dec 21. Hum Gene Ther. 2016. PMID: 26567984
-
A novel surgical approach for intratracheal administration of bioactive agents in a fetal mouse model.J Vis Exp. 2012 Oct 31;(68):4219. doi: 10.3791/4219. J Vis Exp. 2012. PMID: 23149801 Free PMC article.
-
In utero AAV-mediated gene transfer to rabbit pulmonary epithelium.Mol Ther. 2001 Aug;4(2):115-21. doi: 10.1006/mthe.2001.0428. Mol Ther. 2001. PMID: 11482982
-
Gene therapy in cystic fibrosis.Chest. 2001 Sep;120(3 Suppl):124S-131S. doi: 10.1378/chest.120.3_suppl.124s. Chest. 2001. PMID: 11555567 Review.
-
The sheep model of in utero gene therapy.Fetal Diagn Ther. 2004 Jan-Feb;19(1):23-30. doi: 10.1159/000074255. Fetal Diagn Ther. 2004. PMID: 14646413 Review.
Cited by
-
Towards non-invasive monitoring of pathogen-host interactions during Candida albicans biofilm formation using in vivo bioluminescence.Cell Microbiol. 2014 Jan;16(1):115-30. doi: 10.1111/cmi.12184. Epub 2013 Sep 12. Cell Microbiol. 2014. PMID: 23962311 Free PMC article.
-
Fetal hypoplastic lungs have multilineage inflammation that is reversed by amniotic fluid stem cell extracellular vesicle treatment.Sci Adv. 2024 Jul 26;10(30):eadn5405. doi: 10.1126/sciadv.adn5405. Epub 2024 Jul 26. Sci Adv. 2024. PMID: 39058789 Free PMC article.
-
Targeted Gene Delivery through the Respiratory System: Rationale for Intratracheal Gene Transfer.J Cardiovasc Dev Dis. 2019 Feb 15;6(1):8. doi: 10.3390/jcdd6010008. J Cardiovasc Dev Dis. 2019. PMID: 30781363 Free PMC article. Review.
-
A novel translational model for fetoscopic intratracheal delivery of nanoparticles in piglets.Prenat Diagn. 2016 Oct;36(10):926-934. doi: 10.1002/pd.4915. Epub 2016 Sep 18. Prenat Diagn. 2016. PMID: 27567969 Free PMC article.
-
Optical imaging in vivo with a focus on paediatric disease: technical progress, current preclinical and clinical applications and future perspectives.Pediatr Radiol. 2011 Feb;41(2):161-75. doi: 10.1007/s00247-010-1907-0. Epub 2011 Jan 11. Pediatr Radiol. 2011. PMID: 21221568 Free PMC article. Review.
References
-
- Scotet V, Duguépéroux I, Audrézet MP, Blayau M, Boisseau P, Journel H, et al. Prenatal diagnosis of cystic fibrosis: the 18-year experience of Brittany (western France) Prenat Diagn. 2008;28:197–202. - PubMed
-
- Coutelle C, Themis M, Waddington SN, Buckley SM, Gregory LG, Nivsarkar MS, et al. Gene therapy progress and prospects: fetal gene therapy–first proofs of concept–some adverse effects. Gene Ther. 2005;12:1601–1607. - PubMed
-
- Buckley SM, Waddington SN, Jezzard S, Lawrence L, Schneider H, Holder MV, et al. Factors influencing adenovirus-mediated airway transduction in fetal mice. Mol Ther. 2005;12:484–492. - PubMed
-
- Waddington SN, Nivsarkar MS, Mistry AR, Buckley SM, Kemball-Cook G, Mosley KL, et al. Permanent phenotypic correction of hemophilia B in immunocompetent mice by prenatal gene therapy. Blood. 2004;104:2714–2721. - PubMed
-
- Waddington SN, Mitrophanous KA, Ellard FM, Buckley SM, Nivsarkar M, Lawrence L, et al. Long-term transgene expression by administration of a lentivirus-based vector to the fetal circulation of immuno-competent mice. Gene Ther. 2003;10:1234–1240. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

