Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1
- PMID: 20664529
- PMCID: PMC2951565
- DOI: 10.1038/mt.2010.155
Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1
Abstract
Excessive systemic inflammation following trauma, sepsis, or burn could lead to distant organ damage. The transplantation of bone marrow stromal cells or mesenchymal stem cells (MSCs) has been reported to be an effective treatment for several immune disorders by modulating the inflammatory response to injury. We hypothesized that MSCs can dynamically secrete systemic factors that can neutralize the activity of inflammatory cytokines. In this study, we showed that cocultured MSCs are able to decrease nuclear factor κ-B (NFκB) activation in target epithelial cells incubated in inflammatory serum conditions. Proteomic screening revealed a responsive secretion of soluble tumor necrosis factor (TNF) receptor 1 (sTNFR1) when MSCs were exposed to lipopolysaccharide (LPS)-stimulated rat serum. The responsive effect was eliminated when NFκB activation was blocked in MSCs. Intramuscular transplantation of MSCs in LPS-endotoxic rats decreased a panel of inflammatory cytokines and inflammatory infiltration of macrophages and neutrophils in lung, kidney, and liver when compared to controls. These results suggest that improvements of inflammatory responses in animal models after local transplantation of MSCs are at least, in part, explained by the NFκB-dependent secretion of sTNFR1 by MSCs.
Figures

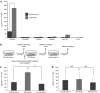

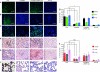
References
-
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD., and, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. - PubMed
-
- Robertson CM., and, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006;8:1382–1389. - PubMed
-
- Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, et al. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–292. - PubMed
-
- Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. - PubMed
-
- Deans KJ, Haley M, Natanson C, Eichacker PQ., and, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58:867–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

