Intestinal epithelia activate anti-viral signaling via intracellular sensing of rotavirus structural components
- PMID: 20664578
- PMCID: PMC2957552
- DOI: 10.1038/mi.2010.39
Intestinal epithelia activate anti-viral signaling via intracellular sensing of rotavirus structural components
Abstract
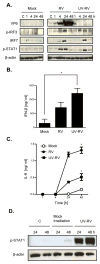
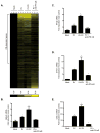
Rotavirus (RV), a leading cause of severe diarrhea, primarily infects intestinal epithelial cells (IECs) causing self-limiting illness. To better understand innate immunity to RV, we sought to define the extent to which IEC activation of anti-viral responses required viral replication or could be recapitulated by inactivated RV or its components. Using model human intestinal epithelia, we observed that RV-induced activation of signaling events and gene expression typically associated with viral infection was largely mimicked by administration of ultraviolet (UV)-inactivated RV. Use of anti-interferon (IFN) neutralizing antibodies revealed that such replication-independent anti-viral gene expression required type I IFN signaling. In contrast, RV-induction of nuclear factor-κB-mediated interleukin-8 expression was dependent on viral replication. The anti-viral gene expression induced by UV-RV was not significantly recapitulated by RV RNA or RV virus-like particles although the latter could enter IEC. Together, these results suggest that RV proteins mediate viral entry into epithelial cells leading to intracellular detection of RV RNA that generates an anti-viral response.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures

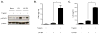
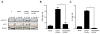
Similar articles
-
Innate immune response to homologous rotavirus infection in the small intestinal villous epithelium at single-cell resolution.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20667-72. doi: 10.1073/pnas.1212188109. Epub 2012 Nov 27. Proc Natl Acad Sci U S A. 2012. PMID: 23188796 Free PMC article.
-
Rotavirus-induced IFN-β promotes anti-viral signaling and apoptosis that modulate viral replication in intestinal epithelial cells.Innate Immun. 2012 Apr;18(2):294-306. doi: 10.1177/1753425911401930. Epub 2011 Jul 6. Innate Immun. 2012. PMID: 21733977
-
Basal interferon signaling and therapeutic use of interferons in controlling rotavirus infection in human intestinal cells and organoids.Sci Rep. 2018 May 29;8(1):8341. doi: 10.1038/s41598-018-26784-9. Sci Rep. 2018. PMID: 29844362 Free PMC article.
-
Rotavirus and reovirus modulation of the interferon response.J Interferon Cytokine Res. 2009 Sep;29(9):559-67. doi: 10.1089/jir.2009.0072. J Interferon Cytokine Res. 2009. PMID: 19694545 Free PMC article. Review.
-
Rotavirus Interactions With Host Intestinal Epithelial Cells.Front Immunol. 2021 Dec 22;12:793841. doi: 10.3389/fimmu.2021.793841. eCollection 2021. Front Immunol. 2021. PMID: 35003114 Free PMC article. Review.
Cited by
-
Innate immune response to homologous rotavirus infection in the small intestinal villous epithelium at single-cell resolution.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20667-72. doi: 10.1073/pnas.1212188109. Epub 2012 Nov 27. Proc Natl Acad Sci U S A. 2012. PMID: 23188796 Free PMC article.
-
Recognition of Reovirus RNAs by the Innate Immune System.Viruses. 2020 Jun 20;12(6):667. doi: 10.3390/v12060667. Viruses. 2020. PMID: 32575691 Free PMC article. Review.
-
Antiviral Activity of Porcine IFN-λ3 and IFN-α against Porcine Rotavirus In Vitro.Molecules. 2022 Jul 18;27(14):4575. doi: 10.3390/molecules27144575. Molecules. 2022. PMID: 35889447 Free PMC article.
-
Early Events in Reovirus Infection Influence Induction of Innate Immune Response.J Virol. 2022 Jul 27;96(14):e0091722. doi: 10.1128/jvi.00917-22. Epub 2022 Jul 6. J Virol. 2022. PMID: 35867576 Free PMC article.
-
Segmented Filamentous Bacteria Prevent and Cure Rotavirus Infection.Cell. 2019 Oct 17;179(3):644-658.e13. doi: 10.1016/j.cell.2019.09.028. Epub 2019 Oct 10. Cell. 2019. PMID: 31607511 Free PMC article.
References
-
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006 Jul 22;368(9532):323–32. - PubMed
-
- Jayaram H, Estes MK, Prasad BV. Emerging themes in rotavirus cell entry, genome organization, transcription and replication. Virus Res. 2004 Apr;101(1):67–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

