The frequency and severity of capecitabine-induced hypertriglyceridaemia in routine clinical practice: a prospective study
- PMID: 20664584
- PMCID: PMC2938254
- DOI: 10.1038/sj.bjc.6605807
The frequency and severity of capecitabine-induced hypertriglyceridaemia in routine clinical practice: a prospective study
Abstract
Background: Capecitabine is known to rarely cause raised serum triglycerides (TG). In our centre, several patients receiving capecitabine developed raised TG levels corresponding to the 'very high risk' category for potentially serious acute pancreatitis.
Methods: A fasting blood lipid screening protocol was introduced into clinical practice for patients receiving capecitabine. Patients with TGs >5 mmol l(-1) were treated and followed up. An 18-month prospective audit was performed to establish the incidence and severity of capecitabine-induced hypertriglyceridaemia (CIHT).
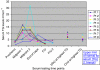
Results: A total of 304 patients received capecitabine for colorectal cancer between January 2008 and June 2009. Of these, 212 patients (70%) were screened and 8 (3.7%) developed clinically significant hypertriglyceridaemia requiring lipid-lowering therapy. Two of the eight patients had diabetes and one had pre-existing dyslipidaemia. One suffered cerebral infarction during chemotherapy. There were no cases of acute pancreatitis. Follow-up showed that serum TGs safely and rapidly returned to normal with appropriate treatment without discontinuation of capecitabine.
Conclusions: This is the first prospective study evaluating CIHT. These results suggest that it should be classed as a 'common' undesired effect of capecitabine. Despite this, the incidence does not justify routine screening in all patients. Targeted screening in those with diabetes or pre-existing hyperlipidaemia is recommended, together with adoption of a clear management policy.
Figures
Comment in
-
Severe hypertriglyceridaemia during treatment with capecitabine.Br J Cancer. 2011 Mar 29;104(7):1238-9. doi: 10.1038/bjc.2011.52. Epub 2011 Mar 8. Br J Cancer. 2011. PMID: 21386842 Free PMC article. No abstract available.
References
-
- Bar-Sela G, Haim N (2009) Uncontrolled hypertriglyceridemia induced by capecitabine: case report and review of the literature. Cancer Chemother Pharmacol 63(5): 779–782 - PubMed
-
- Budman DR, Meropol NJ, Reigner B, Creaven PJ, Lichtman SM, Berghorn E, Behr J, Gordon RJ, Osterwalder B, Griffin T (1998) Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol 16(5): 1795–1802 - PubMed
-
- Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang T-S, Rivera F, Couture F, Sirzen F, Saltz L (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26(12): 2006–2012 - PubMed
-
- Cassidy J, Dirix L, Bissett D, Reigner B, Griffin T, Allman D, Osterwalder B, Van Oosterom AT (1998) A Phase I study of capecitabine in combination with oral leucovorin in patients with intractable solid tumors. Clin Cancer Res 4(11): 2755–2761 - PubMed
-
- Garg R, Angus E, Fincher S (2009) Capecitabine-induced severe hypertriglyceridaemia and diabetes: a case report and review of the literature. Diabet Med 26(12): 1308–1309 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous