Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody-drug conjugate SGN-75
- PMID: 20664585
- PMCID: PMC2938259
- DOI: 10.1038/sj.bjc.6605816
Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody-drug conjugate SGN-75
Abstract
Background: CD70 is an ideal target for antibody-based therapies because of its aberrant high expression in renal carcinomas and non-Hodgkin lymphomas and its highly restricted expression in normal tissues. The expression profiling of CD70 in carcinomas has been limited because of the lack of a CD70-specific reagent that works in formalin-fixed paraffin-embedded (FFPE) tissues.
Methods: We generated murine monoclonal antibodies (mAbs) specific for CD70 and validated their specificity by western blot analysis and developed a protocol for immunohistochemistry on FFPE tissues. CD70+ tumour cell lines were used for testing the anti-tumour activity of the anti-CD70 antibody-drug conjugate, SGN-75.
Results: We report novel detection of CD70 expression in multiple cancers including pancreatic (25%), larynx/pharynx (22%), melanoma (16%), ovarian (15%), lung (10%), and colon (9%). Our results show that pancreatic and ovarian tumour cell lines, which express high levels of endogenous or transfected CD70, are sensitive to the anti-tumour activity of SGN-75 in vitro and in vivo.
Conclusion: Development of murine mAbs for robust and extensive screening of FFPE samples coupled with the detection of anti-tumour activity in novel indications provide rationale for expanding the application of SGN-75 for the treatment of multiple CD70 expressing cancers.
Figures

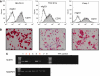
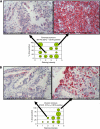

 ) and non-binding ADC (A, squares) have no effect. IC50 for SGN-75 and non-binding ADC are noted. Similarly, no cytotoxicity is observed in the untransfected MiaPaCa-2 control (B, squares). (C and D) In vivo anti-tumour activity of SGN-75 in0-transfected MiaPaCa-2 pancreatic carcinoma tumours in nude mice. Mice treated with SGN-75 (squares) showed a significantly reduced median tumour volume (C) and a higher percent survival (D) when compared with untreated (circles) and non-binding ADC (triangles).
) and non-binding ADC (A, squares) have no effect. IC50 for SGN-75 and non-binding ADC are noted. Similarly, no cytotoxicity is observed in the untransfected MiaPaCa-2 control (B, squares). (C and D) In vivo anti-tumour activity of SGN-75 in0-transfected MiaPaCa-2 pancreatic carcinoma tumours in nude mice. Mice treated with SGN-75 (squares) showed a significantly reduced median tumour volume (C) and a higher percent survival (D) when compared with untreated (circles) and non-binding ADC (triangles).Similar articles
-
CD70 antibody-drug conjugate as a potential therapeutic agent for uterine leiomyosarcoma.Am J Obstet Gynecol. 2021 Feb;224(2):197.e1-197.e23. doi: 10.1016/j.ajog.2020.08.028. Epub 2020 Aug 19. Am J Obstet Gynecol. 2021. PMID: 32822640
-
Engineered anti-CD70 antibody-drug conjugate with increased therapeutic index.Mol Cancer Ther. 2008 Sep;7(9):2913-23. doi: 10.1158/1535-7163.MCT-08-0295. Mol Cancer Ther. 2008. PMID: 18790772
-
CD70 antibody-drug conjugate: A potential novel therapeutic agent for ovarian cancer.Cancer Sci. 2021 Sep;112(9):3655-3668. doi: 10.1111/cas.15027. Epub 2021 Jul 8. Cancer Sci. 2021. PMID: 34117815 Free PMC article.
-
CD70: An Emerging Anticancer Target in Renal Cell Carcinoma and Beyond.Annu Rev Med. 2025 Jan;76(1):257-266. doi: 10.1146/annurev-med-070623-045906. Epub 2025 Jan 16. Annu Rev Med. 2025. PMID: 39570653 Review.
-
[Immunoconjugates, drug-armed antibodies to fight against cancer].Med Sci (Paris). 2009 Dec;25(12):1046-52. doi: 10.1051/medsci/200925121046. Med Sci (Paris). 2009. PMID: 20035677 Review. French.
Cited by
-
CD70-restricted specific activation of TRAILR1 or TRAILR2 using scFv-targeted TRAIL mutants.Cell Death Dis. 2014 Jan 30;5(1):e1035. doi: 10.1038/cddis.2013.555. Cell Death Dis. 2014. PMID: 24481449 Free PMC article.
-
Mechanisms of Immune Evasion and Immune Modulation by Lymphoma Cells.Front Oncol. 2018 Mar 7;8:54. doi: 10.3389/fonc.2018.00054. eCollection 2018. Front Oncol. 2018. PMID: 29564225 Free PMC article. Review.
-
Generation of a CD70-Specific Fusion Nanobody with IgG Recruiting Capacity for Tumor Killing.Int J Nanomedicine. 2023 Jun 19;18:3325-3338. doi: 10.2147/IJN.S410281. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37361386 Free PMC article.
-
CD70, a novel target of CAR T-cell therapy for gliomas.Neuro Oncol. 2018 Jan 10;20(1):55-65. doi: 10.1093/neuonc/nox116. Neuro Oncol. 2018. PMID: 28651374 Free PMC article.
-
Increased CD70 expression is associated with clinical resistance to cisplatin-based chemotherapy and poor survival in advanced ovarian carcinomas.Onco Targets Ther. 2013 Jun 5;6:615-9. doi: 10.2147/OTT.S44445. Print 2013. Onco Targets Ther. 2013. PMID: 23776334 Free PMC article.
References
-
- Agathanggelou A, Niedobitek G, Chen R, Nicholls J, Yin W, Young LS (1995) Expression of immune regulatory molecules in Epstein-Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma. Evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am J Pathol 147: 1152–1160 - PMC - PubMed
-
- Agematsu K, Nagumo H, Oguchi Y, Nakazawa T, Fukushima K, Yasui K, Ito S, Kobata T, Morimoto C, Komiyama A (1998) Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood 91: 173–180 - PubMed
-
- Aggarwal S, He T, Fitzhugh W, Rosenthal K, Feild B, Heidbrink J, Mesmer D, Ruben SM, Moore PA (2009) Immune modulator CD70 as a potential cisplatin resistance predictive marker in ovarian cancer. Gynecol Oncol 115: 430–437 - PubMed
-
- Al Saati TMC, Caspar S, Hounieu H, Brousset P, Magaud JP, Thomsen M, Pallesen G, Gorguet B, Chittal S, Delsol G (1989) Production of Two mAb Identifying a Novel Activation Antigen (CDw70), Using Spleen Cels from Nude Mice Bearing HLY-1 Cell Line. Oxford University Press: Oxford, United Kingdom
-
- Alley SC, Zhang X, Okeley NM, Anderson M, Law CL, Senter PD, Benjamin DR (2009) The pharmacologic basis for antibody-auristatin conjugate activity. J Pharmacol Exp Ther 330: 932–938 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

