A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands
- PMID: 20664589
- PMCID: PMC2938258
- DOI: 10.1038/sj.bjc.6605815
A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands
Abstract
Background: Most men with elevated levels of prostate-specific antigen (PSA) do not have prostate cancer, leading to a large number of unnecessary biopsies. A statistical model based on a panel of four kallikreins has been shown to predict the outcome of a first prostate biopsy. In this study, we apply the model to an independent data set of men with previous negative biopsy but persistently elevated PSA.
Methods: The study cohort consisted of 925 men with a previous negative prostate biopsy and elevated PSA (>or=3 ng ml(-1)), with 110 prostate cancers detected (12%). A previously published statistical model was applied, with recalibration to reflect the lower positive biopsy rates on rebiopsy.
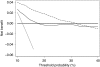
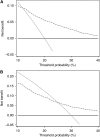
Results: The full-kallikrein panel had higher discriminative accuracy than PSA and DRE alone, with area under the curve (AUC) improving from 0.58 (95% confidence interval (CI): 0.52, 0.64) to 0.68 (95% CI: 0.62, 0.74), P<0.001, and high-grade cancer (Gleason >or=7) at biopsy with AUC improving from 0.76 (95% CI: 0.64, 0.89) to 0.87 (95% CI: 0.81, 0.94), P=0.003). Application of the panel to 1000 men with persistently elevated PSA after initial negative biopsy, at a 15% risk threshold would reduce the number of biopsies by 712; would miss (or delay) the diagnosis of 53 cancers, of which only 3 would be Gleason 7 and the rest Gleason 6 or less.
Conclusions: Our data constitute an external validation of a previously published model. The four-kallikrein panel predicts the result of repeat prostate biopsy in men with elevated PSA while dramatically decreasing unnecessary biopsies.
Conflict of interest statement
Dr H Lilja holds patents for free PSA and hK2 assays, and, with Dr Kim Pettersson, is named as co-inventor on a patent application for intact/nicked PSA assays.
Figures
References
-
- Benecchi L, Pieri AM, Melissari M, Potenzoni M, Pastizzaro CD (2008) A novel nomogram to predict the probability of prostate cancer on repeat biopsy. J Urol 180(1): 146–149 - PubMed
-
- Catalona WJ, Beiser JA, Smith DS (1997) Serum free prostate specific antigen and prostate specific antigen density measurements for predicting cancer in men with prior negative prostatic biopsies. J Urol 158(6): 2162–2167 - PubMed
-
- Chun FK, Briganti A, Graefen M, Porter C, Montorsi F, Haese A, Scattoni V, Borden L, Steuber T, Salonia A, Schlomm T, Latchemsetty K, Walz J, Kim J, Eichelberg C, Currlin E, Ahyai SA, Erbersdobler A, Valiquette L, Heinzer H, Rigatti P, Huland H, Karakiewicz PI (2007) Development and external validation of an extended repeat biopsy nomogram. J Urol 177(2): 510–515 - PubMed
-
- Djavan B, Zlotta A, Remzi M, Ghawidel K, Basharkhah A, Schulman CC, Marberger M (2000) Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1,051 men. J Urol 163(4): 1144–1148; discussion 1148–9 - PubMed
-
- Durkan GC, Greene DR (1999) Elevated serum prostate specific antigen levels in conjunction with an initial prostatic biopsy negative for carcinoma: who should undergo a repeat biopsy? BJU Int 83(1): 34–38 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

