The endocannabinoid system links gut microbiota to adipogenesis
- PMID: 20664638
- PMCID: PMC2925525
- DOI: 10.1038/msb.2010.46
The endocannabinoid system links gut microbiota to adipogenesis
Abstract
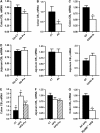
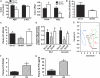
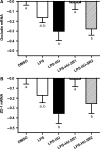
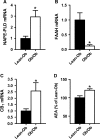
Obesity is characterised by altered gut microbiota, low-grade inflammation and increased endocannabinoid (eCB) system tone; however, a clear connection between gut microbiota and eCB signalling has yet to be confirmed. Here, we report that gut microbiota modulate the intestinal eCB system tone, which in turn regulates gut permeability and plasma lipopolysaccharide (LPS) levels. The impact of the increased plasma LPS levels and eCB system tone found in obesity on adipose tissue metabolism (e.g. differentiation and lipogenesis) remains unknown. By interfering with the eCB system using CB(1) agonist and antagonist in lean and obese mouse models, we found that the eCB system controls gut permeability and adipogenesis. We also show that LPS acts as a master switch to control adipose tissue metabolism both in vivo and ex vivo by blocking cannabinoid-driven adipogenesis. These data indicate that gut microbiota determine adipose tissue physiology through LPS-eCB system regulatory loops and may have critical functions in adipose tissue plasticity during obesity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

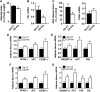

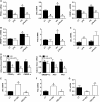

References
-
- Bennetzen MF, Nielsen TS, Paulsen SK, Bendix J, Fisker S, Jessen N, Lund S, Richelsen B, Pedersen SB (2010) Reduced cannabinoid receptor 1 protein in subcutaneous adipose tissue of obese. Eur J Clin Invest 40: 121–126 - PubMed
-
- Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U (2008) Cannabinoid type 1 receptor: another arrow in the adipocytes' bow. J Neuroendocrinol 20(Suppl 1): 130–138 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

