NPR-B natriuretic peptide receptors in human corneal epithelium: mRNA, immunohistochemistochemical, protein, and biochemical pharmacology studies
- PMID: 20664698
- PMCID: PMC2903464
NPR-B natriuretic peptide receptors in human corneal epithelium: mRNA, immunohistochemistochemical, protein, and biochemical pharmacology studies
Abstract
Purpose: To demonstrate the presence of natriuretic peptide receptors (NPRs) in primary human corneal epithelial cells (p-CEPI), SV40-immortalized CEPI cells (CEPI-17-CL4) and in human corneal epithelium, and to define the pharmacology of natriuretic peptide (NP)-induced cGMP accumulation.
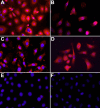
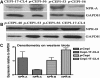
Methods: NPR presence was shown by RT-PCR, western blot analysis, and indirect immunofluoresence. cGMP accumulation was determined using an enzyme immunoassay.
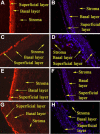
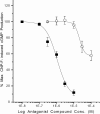
Results: p-CEPI and CEPI-17-CL4 cells expressed mRNAs for NPR-A and NPR-B. Proteins for both NPRs were present in these cells and in human corneal epithelium. C-type NP (CNP), atrial NP (ANP) and brain NP (BNP) stimulated the accumulation of cGMP in a concentration-dependent manner in p-CEPI cells (potency; EC(50s)): CNP (1-53 amino acids) EC(50)=24+/-5 nM; CNP fragment (32-53 amino acids) EC(50)=51+/-8 nM; ANP (1-28 amino acids) EC(50)=>10 microM; BNP (32 amino acids) EC(50)>10 microM (all n=3-4). While the NPs were generally more potent in the CEPI-17-CL4 cells than in p-CEPI cells (n=4-9; p<0.01), the rank order of potency of the peptides was essentially the same in both cell types. Effects of CNP fragment in p-CEPI and CEPI-17-CL4 cells were potently blocked by HS-142-1, an NPR-B receptor subtype-selective antagonist (K(i)=0.25+/-0.05 microM in CEPI-CL4-17; K(i)=0.44+/-0.09 microM in p-CEPIs; n=6-7) but less so by an NPR-A receptor antagonist, isatin (K(i)=5.3-7.8 microM, n=3-7).
Conclusions: Our studies showed the presence of NPR-A and NPR-B (mRNAs and protein) in p-CEPI and CEPI-17-CL4 cells and in human corneal epithelial tissue. However, detailed pharmacological studies revealed NPR-B to be the predominant functionally active receptor in both cell-types whose activation leads to the generation of cGMP. While the physiologic role(s) of the NP system in corneal function remains to be delineated, our multidisciplinary findings pave the way for such future investigations.
Figures

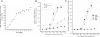
References
-
- deBold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–70. - PubMed
-
- Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. - PubMed
-
- Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–70. - PubMed
-
- Drewett JG, Garbers DL. The family of guanylyl cyclase receptors and their ligands. Endocr Rev. 1994;15:135–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
