Efficacy and safety of sunitinib on metastatic renal cell carcinoma: a single-institution experience
- PMID: 20664776
- PMCID: PMC2907492
- DOI: 10.4111/kju.2010.51.7.450
Efficacy and safety of sunitinib on metastatic renal cell carcinoma: a single-institution experience
Abstract
Purpose: We assessed the efficacy and safety of the tyrosine kinase inhibitor sunitinib in Korean patients with metastatic renal cell carcinoma (mRCC).
Materials and methods: Between September 2007 and December 2009, all twenty-one patients who had mRCC with a clear-cell component were retrospectively reviewed. Sunitinib was administered orally at a dose of 50 mg daily until disease progression or intolerance to treatment occurred. The primary end point of this study was the objective tumor response assessed by Response Evaluation Criteria in Solid Tumors (RECIST), and the secondary end points were progression-free survival (PFS) and overall survival (OS) rates as well as assessment of adverse effects.
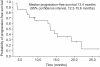
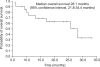
Results: After a median of 17.4 months (range, 5.7-33.1 months) of treatment, 11 patients (52.4%) had an objective response with a complete response in 1 patient (4.8%), and a partial response in 10 patients (47.6%) as the best tumor response. The median PFS was 13.4 months (95% confidence interval [CI], range, 12.3-14.5 months), and the median OS was 28.1 months (95% CI, 21.8-34.4 months). All patients experienced adverse events of some sort, but the studied treatment protocol was well tolerated and most patients experienced reversible grade 1 or 2 toxicities.
Conclusions: Sunitinib was efficacious in the treatment of metastatic clear-cell RCC, and was well tolerated in Korean patients. Although sunitinib treatment-related adverse events such as hand-foot syndrome and facial/generalized edema were observed with a higher incidence than in Western trials, they were mainly mild to moderate, and readily managed.
Keywords: Neoplasm metastasis; Renal cell carcinoma; Sunitinib.
Conflict of interest statement
The authors have nothing to disclose.
Figures
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23:202–212. - PubMed
-
- Motzer RJ, Russo P, Nanus DM, Berg WJ. Renal cell carcinoma. Curr Probl Cancer. 1997;21:185–232. - PubMed
-
- Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. - PubMed
-
- Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O'Connell M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824–832. - PubMed
LinkOut - more resources
Full Text Sources