Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes
- PMID: 20664791
- PMCID: PMC2904800
- DOI: 10.1371/journal.ppat.1001003
Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes
Erratum in
-
Correction: Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes.PLoS Pathog. 2010 Aug 10;6(8):10.1371/annotation/738ac91f-8c41-4bf5-9a39-bddf0b777a89. doi: 10.1371/annotation/738ac91f-8c41-4bf5-9a39-bddf0b777a89. PLoS Pathog. 2010. PMID: 20714345 Free PMC article.
Abstract
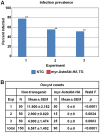
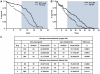
Malaria (Plasmodium spp.) kills nearly one million people annually and this number will likely increase as drug and insecticide resistance reduces the effectiveness of current control strategies. The most important human malaria parasite, Plasmodium falciparum, undergoes a complex developmental cycle in the mosquito that takes approximately two weeks and begins with the invasion of the mosquito midgut. Here, we demonstrate that increased Akt signaling in the mosquito midgut disrupts parasite development and concurrently reduces the duration that mosquitoes are infective to humans. Specifically, we found that increased Akt signaling in the midgut of heterozygous Anopheles stephensi reduced the number of infected mosquitoes by 60-99%. Of those mosquitoes that were infected, we observed a 75-99% reduction in parasite load. In homozygous mosquitoes with increased Akt signaling parasite infection was completely blocked. The increase in midgut-specific Akt signaling also led to an 18-20% reduction in the average mosquito lifespan. Thus, activation of Akt signaling reduced the number of infected mosquitoes, the number of malaria parasites per infected mosquito, and the duration of mosquito infectivity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

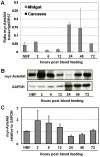
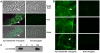
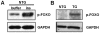
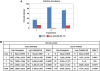
References
-
- Roll Back Malaria/WHO/UNICEF. World Malaria Report 2008. World Health Organization 2008
-
- Quraishi MS, Esghi N, Faghih MA. Flight range, lengths of gonotrophic cycles, and longevity of P-32-labeled Anopheles stephensi mysorensis. J Econ Entomol. 1966;59:50–55. - PubMed
-
- Reisen WK, Aslamkhan M. A release-recapture experiment with the malaria vector, Anopheles stephensi liston, with observations on dispersal, survivorship, population size, gonotrophic rhythm and mating behaviour. Ann Trop Med Parasitol. 1979;73:251–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

