Recent progress in the structure determination of GPCRs, a membrane protein family with high potential as pharmaceutical targets
- PMID: 20665265
- PMCID: PMC2973844
- DOI: 10.1007/978-1-60761-762-4_8
Recent progress in the structure determination of GPCRs, a membrane protein family with high potential as pharmaceutical targets
Abstract
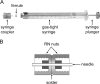
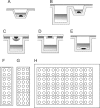
G protein-coupled receptors (GPCRs) constitute a highly diverse and ubiquitous family of integral membrane proteins, transmitting signals inside the cells in response to an assortment of disparate extracellular stimuli. Their strategic location on the cell surface and their involvement in crucial cellular and physiological processes turn these receptors into highly important pharmaceutical targets. Recent technological developments aimed at stabilization and crystallization of these receptors have led to significant breakthroughs in GPCR structure determination efforts. One of the successful approaches involved receptor stabilization with the help of a fusion partner combined with crystallization in lipidic cubic phase (LCP). The success of using an LCP matrix for crystallization is generally attributed to the creation of a more native, membrane-like stabilizing environment for GPCRs just prior to nucleation and to the formation of type I crystal lattices, thus generating highly ordered and strongly diffracting crystals. Here we describe protocols for reconstituting purified GPCRs in LCP, performing pre-crystallization assays, setting up crystallization trials in manual mode, detecting crystallization hits, optimizing crystallization conditions, harvesting, and collecting crystallographic data The protocols provide a sensible framework for approaching crystallization of stabilized GPCRs in LCP, however, as in any crystallization experiment, extensive screening and optimization of crystallization conditions as well as optimization of protein construct and purification steps are required. The process remains risky and these protocols do not necessarily guarantee success.
Figures




References
-
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. - PubMed
-
- Rubenstein K. GPCRs: Dawn of a New Era? Cambridge Healthtech Institute; Needham: 2008.
-
- Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1:761–82. - PubMed
-
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. - PubMed
-
- Bosier B, Hermans E. Versatility of GPCR recognition by drugs: from biological implications to therapeutic relevance. Trends Pharmacol Sci. 2007;228:438–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

