Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes
- PMID: 20665559
- PMCID: PMC3580049
- DOI: 10.1002/glia.21052
Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes
Abstract
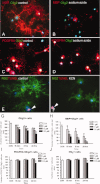
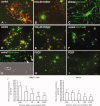
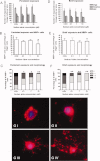
Oligodendrocyte lineage cells are susceptible to a variety of insults including hypoxia, excitotoxicity, and reactive oxygen species. Demyelination is a well-recognized feature of several CNS disorders including multiple sclerosis, white matter strokes, progressive multifocal leukoencephalopathy, and disorders due to mitochondrial DNA mutations. Although mitochondria have been implicated in the demise of oligodendrocyte lineage cells, the consequences of mitochondrial respiratory chain defects have not been examined. We determine the in vitro impact of established inhibitors of mitochondrial respiratory chain complex IV or cytochrome c oxidase on oligodendrocyte progenitor cells (OPCs) and mature oligodendrocytes as well as on differentiation capacity of OPCs from P0 rat. Injury to mature oligodendrocytes following complex IV inhibition was significantly greater than to OPCs, judged by cell detachment and mitochondrial membrane potential (MMP) changes, although viability of cells that remained attached was not compromised. Active mitochondria were abundant in processes of differentiated oligodendrocytes and MMP was significantly greater in differentiated oligodendrocytes than OPCs. MMP dissipated following complex IV inhibition in oligodendrocytes. Furthermore, complex IV inhibition impaired process formation within oligodendrocyte lineage cells. Injury to and impaired process formation of oligodendrocytes following complex IV inhibition has potentially important implications for the pathogenesis and repair of CNS myelin disorders.
© 2010 Wiley-Liss, Inc.
Figures

References
-
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Bruck W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. - PubMed
-
- Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: Contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–1149. - PubMed
-
- Almazan G, Liu HN, Khorchid A, Sundararajan S, Martinez-Bermudez AK, Chemtob S. Exposure of developing oligodendrocytes to cadmium causes HSP72 induction, free radical generation, reduction in glutathione levels, and cell death. Free Radic Biol Med. 2000;29:858–869. - PubMed
-
- Andrews T, Zhang P, Bhat NR. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

