Linkage isomerization in heme-NOx compounds: understanding NO, nitrite, and hyponitrite interactions with iron porphyrins
- PMID: 20666385
- PMCID: PMC3998715
- DOI: 10.1021/ic902423v
Linkage isomerization in heme-NOx compounds: understanding NO, nitrite, and hyponitrite interactions with iron porphyrins
Abstract
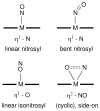
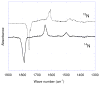
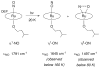
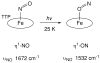
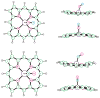
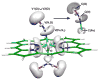
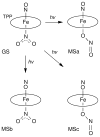
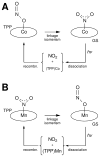
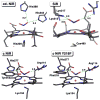
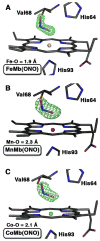
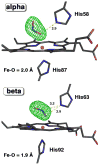
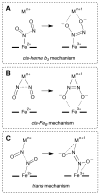
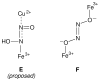
Nitric oxide (NO) and its derivatives such as nitrite and hyponitrite are biologically important species of relevance to human health. Much of their physiological relevance stems from their interactions with the iron centers in heme proteins. The chemical reactivities displayed by the heme-NOx species (NOx = NO, nitrite, hyponitrite) are a function of the binding modes of the NOx ligands. Hence, an understanding of the types of binding modes extant in heme-NOx compounds is important if we are to unravel the inherent chemical properties of these NOx metabolites. In this Forum Article, the experimentally characterized linkage isomers of heme-NOx models and proteins are presented and reviewed. Nitrosyl linkage isomers of synthetic iron and ruthenium porphyrins have been generated by photolysis at low temperatures and characterized by spectroscopy and density functional theory calculations. Nitrite linkage isomers in synthetic metalloporphyrin derivatives have been generated from photolysis experiments and in low-temperature matrices. In the case of nitrite adducts of heme proteins, both N and O binding have been determined crystallographically, and the role of the distal H-bonding residue in myoglobin in directing the O-binding mode of nitrite has been explored using mutagenesis. To date, only one synthetic metalloporphyrin complex containing a hyponitrite ligand (displaying an O-binding mode) has been characterized by crystallography. This is contrasted with other hyponitrite binding modes experimentally determined for coordination compounds and computationally for NO reductase enzymes. Although linkage isomerism in heme-NOx derivatives is still in its infancy, opportunities now exist for a detailed exploration of the existence and stabilities of the metastable states in both heme models and heme proteins.
Figures
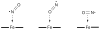
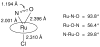
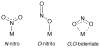
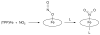
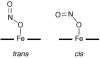

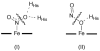
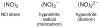
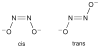
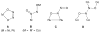
Similar articles
-
Experimental and density functional theoretical investigations of linkage isomerism in six-coordinate FeNO(6) iron porphyrins with axial nitrosyl and nitro ligands.J Am Chem Soc. 2006 Feb 15;128(6):2093-104. doi: 10.1021/ja0567891. J Am Chem Soc. 2006. PMID: 16464112
-
Iron(II) porphyrins induced conversion of nitrite into nitric oxide: A computational study.J Inorg Biochem. 2015 Sep;150:126-32. doi: 10.1016/j.jinorgbio.2015.06.005. Epub 2015 Jun 22. J Inorg Biochem. 2015. PMID: 26112152
-
The distal pocket histidine residue in horse heart myoglobin directs the O-binding mode of nitrite to the heme iron.J Am Chem Soc. 2009 Dec 23;131(50):18119-28. doi: 10.1021/ja904726q. J Am Chem Soc. 2009. PMID: 19924902 Free PMC article.
-
NO and NOx interactions with group 8 metalloporphyrins.J Inorg Biochem. 2005 Jan;99(1):151-65. doi: 10.1016/j.jinorgbio.2004.10.002. J Inorg Biochem. 2005. PMID: 15598499 Review.
-
Solid-state structures of metalloporphyrin NO(x )compounds.Chem Rev. 2002 Apr;102(4):1067-90. doi: 10.1021/cr000080p. Chem Rev. 2002. PMID: 11942787 Review. No abstract available.
Cited by
-
Impact of Nitrate on the Removal of Pollutants from Water in Reducing Gas-Based Membrane Biofilm Reactors: A Review.Membranes (Basel). 2024 May 9;14(5):109. doi: 10.3390/membranes14050109. Membranes (Basel). 2024. PMID: 38786943 Free PMC article. Review.
-
Elucidation of the heme active site electronic structure affecting the unprecedented nitrite dismutase activity of the ferriheme b proteins, the nitrophorins.Chem Sci. 2016 Aug 1;7(8):5332-5340. doi: 10.1039/c6sc01019a. Epub 2016 Apr 25. Chem Sci. 2016. PMID: 30155185 Free PMC article.
-
Solvent Composition Drives the Rebinding Kinetics of Nitric Oxide to Microperoxidase.Sci Rep. 2018 Mar 27;8(1):5281. doi: 10.1038/s41598-018-22944-z. Sci Rep. 2018. PMID: 29588445 Free PMC article.
-
Complex Interplay of Heme-Copper Oxidases with Nitrite and Nitric Oxide.Int J Mol Sci. 2022 Jan 17;23(2):979. doi: 10.3390/ijms23020979. Int J Mol Sci. 2022. PMID: 35055165 Free PMC article. Review.
-
The reaction of rhenium nitrosyl with a sterically hindered NHC-carbene.Dalton Trans. 2022 Jan 25;51(4):1521-1526. doi: 10.1039/d1dt03966k. Dalton Trans. 2022. PMID: 34989739 Free PMC article.
References
-
- Cheng L, Richter-Addo GB. The Porphyrin Handbook. In: Guilard R, Smith K, Kadish KM, editors. Biochemistry and Binding: Activation of Small Molecules. Vol. 4. Academic Press; New York: 2000. pp. 219–291.
-
- Richter-Addo GB, Legzdins P. Metal Nitrosyls. Oxford University Press; New York: 1992.
-
- Carducci MD, Pressprich MR, Coppens P. J Am Chem Soc. 1997;119:2669–2678.
-
- Fomitchev DV, Coppens P. Inorg Chem. 1996;35:7021–7026. - PubMed
-
- Fomitchev DV, Coppens P. Comments Inorg Chem. 1999;21:131–148.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

