Linkage isomerization in heme-NOx compounds: understanding NO, nitrite, and hyponitrite interactions with iron porphyrins
- PMID: 20666385
- PMCID: PMC3998715
- DOI: 10.1021/ic902423v
Linkage isomerization in heme-NOx compounds: understanding NO, nitrite, and hyponitrite interactions with iron porphyrins
Abstract
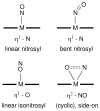
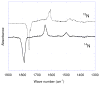
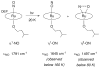
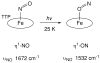
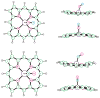
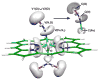
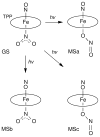
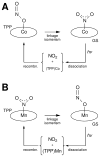
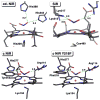
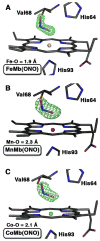
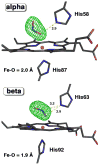
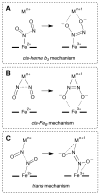
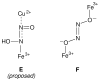
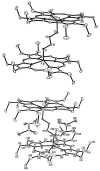
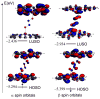
Nitric oxide (NO) and its derivatives such as nitrite and hyponitrite are biologically important species of relevance to human health. Much of their physiological relevance stems from their interactions with the iron centers in heme proteins. The chemical reactivities displayed by the heme-NOx species (NOx = NO, nitrite, hyponitrite) are a function of the binding modes of the NOx ligands. Hence, an understanding of the types of binding modes extant in heme-NOx compounds is important if we are to unravel the inherent chemical properties of these NOx metabolites. In this Forum Article, the experimentally characterized linkage isomers of heme-NOx models and proteins are presented and reviewed. Nitrosyl linkage isomers of synthetic iron and ruthenium porphyrins have been generated by photolysis at low temperatures and characterized by spectroscopy and density functional theory calculations. Nitrite linkage isomers in synthetic metalloporphyrin derivatives have been generated from photolysis experiments and in low-temperature matrices. In the case of nitrite adducts of heme proteins, both N and O binding have been determined crystallographically, and the role of the distal H-bonding residue in myoglobin in directing the O-binding mode of nitrite has been explored using mutagenesis. To date, only one synthetic metalloporphyrin complex containing a hyponitrite ligand (displaying an O-binding mode) has been characterized by crystallography. This is contrasted with other hyponitrite binding modes experimentally determined for coordination compounds and computationally for NO reductase enzymes. Although linkage isomerism in heme-NOx derivatives is still in its infancy, opportunities now exist for a detailed exploration of the existence and stabilities of the metastable states in both heme models and heme proteins.
Figures
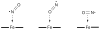
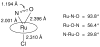
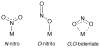
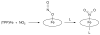
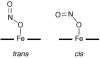
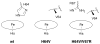

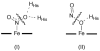
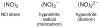
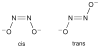
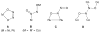
References
-
- Cheng L, Richter-Addo GB. The Porphyrin Handbook. In: Guilard R, Smith K, Kadish KM, editors. Biochemistry and Binding: Activation of Small Molecules. Vol. 4. Academic Press; New York: 2000. pp. 219–291.
-
- Richter-Addo GB, Legzdins P. Metal Nitrosyls. Oxford University Press; New York: 1992.
-
- Carducci MD, Pressprich MR, Coppens P. J Am Chem Soc. 1997;119:2669–2678.
-
- Fomitchev DV, Coppens P. Inorg Chem. 1996;35:7021–7026. - PubMed
-
- Fomitchev DV, Coppens P. Comments Inorg Chem. 1999;21:131–148.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

