Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels
- PMID: 20666585
- PMCID: PMC2965196
- DOI: 10.1089/ten.tea.2010.0233
Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels
Abstract
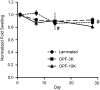
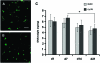
To inform future efforts in tendon/ligament tissue engineering, our laboratory has developed a well-controlled model system with the ability to alter both external tensile loading parameters and local biochemical cues to better understand marrow stromal cell differentiation in response to both stimuli concurrently. In particular, the synthetic, poly(ethylene glycol)-based hydrogel material oligo(poly(ethylene glycol) fumarate) (OPF) has been explored as a cell carrier for this system. This biomaterial can be tailored to present covalently incorporated bioactive moieties and can be loaded in our custom cyclic tensile bioreactor for up to 28 days with no loss of material integrity. Human marrow stromal cells encapsulated in these OPF hydrogels were cultured (21 days) under cyclic tensile strain (10%, 1 Hz, 3 h of strain followed by 3 h without) or at 0% strain. No difference was observed in cell number due to mechanical stimulation or across time (n = 4), with cells remaining viable (n = 4) through 21 days. Cyclic strain significantly upregulated all tendon/ligament fibroblastic genes examined (collagen I, collagen III, and tenascin-C) by day 21 (n ≥ 6), whereas genes for other pathways (osteogenic, chondrogenic, and adipogenic) did not increase. After 21 days, the presence of collagen I and tenascin-C was observed via immunostaining (n = 2). This study demonstrates the utility of this hydrogel/bioreactor system as a versatile, yet well-controlled, model environment to study marrow stromal cell differentiation toward the tendon/ligament phenotype under a variety of conditions.
Figures

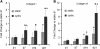
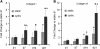

Similar articles
-
Modulation of mesenchymal stem cell shape in enzyme-sensitive hydrogels is decoupled from upregulation of fibroblast markers under cyclic tension.Tissue Eng Part A. 2012 Nov;18(21-22):2365-75. doi: 10.1089/ten.TEA.2011.0727. Epub 2012 Jul 25. Tissue Eng Part A. 2012. PMID: 22703182 Free PMC article.
-
In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels.J Biomed Mater Res A. 2004 Aug 1;70(2):235-44. doi: 10.1002/jbm.a.30064. J Biomed Mater Res A. 2004. PMID: 15227668
-
The effects of intermittent dynamic loading on chondrogenic and osteogenic differentiation of human marrow stromal cells encapsulated in RGD-modified poly(ethylene glycol) hydrogels.Acta Biomater. 2011 Nov;7(11):3829-40. doi: 10.1016/j.actbio.2011.06.031. Epub 2011 Jun 26. Acta Biomater. 2011. PMID: 21742067
-
Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro.Biomacromolecules. 2004 Jan-Feb;5(1):5-10. doi: 10.1021/bm030067p. Biomacromolecules. 2004. PMID: 14715001
-
Survival, proliferation and differentiation enhancement of neural stem cells cultured in three-dimensional polyethylene glycol-RGD hydrogel with tenascin.J Tissue Eng Regen Med. 2016 Mar;10(3):199-208. doi: 10.1002/term.1958. Epub 2014 Oct 13. J Tissue Eng Regen Med. 2016. PMID: 25312025
Cited by
-
In Vitro Innovation of Tendon Tissue Engineering Strategies.Int J Mol Sci. 2020 Sep 14;21(18):6726. doi: 10.3390/ijms21186726. Int J Mol Sci. 2020. PMID: 32937830 Free PMC article. Review.
-
Strategies for Annulus Fibrosus Regeneration: From Biological Therapies to Tissue Engineering.Front Bioeng Biotechnol. 2018 Jul 10;6:90. doi: 10.3389/fbioe.2018.00090. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30042942 Free PMC article. Review.
-
Biomaterials-Based Strategies for the Engineering of Mechanically Active Soft Tissues.MRS Commun. 2012 Jun 1;2(2):31-39. doi: 10.1557/mrc.2012.4. MRS Commun. 2012. PMID: 25250199 Free PMC article.
-
The influence of cyclic tensile strain on multi-compartment collagen-GAG scaffolds for tendon-bone junction repair.Connect Tissue Res. 2019 Nov;60(6):530-543. doi: 10.1080/03008207.2019.1601183. Epub 2019 Apr 22. Connect Tissue Res. 2019. PMID: 31007094 Free PMC article.
-
Cyclic Tensile Strain Can Play a Role in Directing both Intramembranous and Endochondral Ossification of Mesenchymal Stem Cells.Front Bioeng Biotechnol. 2017 Nov 27;5:73. doi: 10.3389/fbioe.2017.00073. eCollection 2017. Front Bioeng Biotechnol. 2017. PMID: 29230389 Free PMC article.
References
-
- Butler D.L. Dressler M. Awad H. Functional tissue engineering: assessment of function in tendon and ligament repair. In: Guilak F., editor; Butler D., editor; Goldstein S., editor; Mooney D., editor. Functional Tissue Engineering. New York: Springer; 2003. pp. 213–226.
-
- Mechanical properties of ligament and tendon. In: Martin R., editor; Burr D., editor; Sharkey N., editor. Skeletal Tissue Mechanics. New York, NY: Springer; 1998. pp. 309–346.
-
- Louie L. Yannas I.V. Spector M. Tissue engineered tendon. In: Patrick C.W. Jr., editor; Mikos A.G., editor; McIntire L.V., editor. Frontiers in Tissue Engineering. New York: Elsevier Science Inc.; 1998. pp. 412–442.
-
- Kessler M.A. Behrend H. Henz S. Stutz G. Rukavina A. Kuster M.S. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16:442. - PubMed
-
- Deng D. Liu W. Xu F. Yang Y. Zhou G. Zhang W.J. Cui L. Cao Y. Engineering human neo-tendon tissue in vitro with human dermal fibroblasts under static mechanical strain. Biomaterials. 2009;30:6724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

