Interference with histidyl-tRNA synthetase by a CRISPR spacer sequence as a factor in the evolution of Pelobacter carbinolicus
- PMID: 20667132
- PMCID: PMC2923632
- DOI: 10.1186/1471-2148-10-230
Interference with histidyl-tRNA synthetase by a CRISPR spacer sequence as a factor in the evolution of Pelobacter carbinolicus
Abstract
Background: Pelobacter carbinolicus, a bacterium of the family Geobacteraceae, cannot reduce Fe(III) directly or produce electricity like its relatives. How P. carbinolicus evolved is an intriguing problem. The genome of P. carbinolicus contains clustered regularly interspaced short palindromic repeats (CRISPR) separated by unique spacer sequences, which recent studies have shown to produce RNA molecules that interfere with genes containing identical sequences.
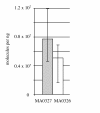
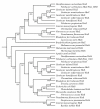
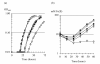
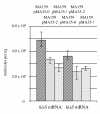
Results: CRISPR spacer #1, which matches a sequence within hisS, the histidyl-tRNA synthetase gene of P. carbinolicus, was shown to be expressed. Phylogenetic analysis and genetics demonstrated that a gene paralogous to hisS in the genomes of Geobacteraceae is unlikely to compensate for interference with hisS. Spacer #1 inhibited growth of a transgenic strain of Geobacter sulfurreducens in which the native hisS was replaced with that of P. carbinolicus. The prediction that interference with hisS would result in an attenuated histidyl-tRNA pool insufficient for translation of proteins with multiple closely spaced histidines, predisposing them to mutation and elimination during evolution, was investigated by comparative genomics of P. carbinolicus and related species. Several ancestral genes with high histidine demand have been lost or modified in the P. carbinolicus lineage, providing an explanation for its physiological differences from other Geobacteraceae.
Conclusions: The disappearance of multiheme c-type cytochromes and other genes typical of a metal-respiring ancestor from the P. carbinolicus lineage may be the consequence of spacer #1 interfering with hisS, a condition that can be reproduced in a heterologous host. This is the first successful co-introduction of an active CRISPR spacer and its target in the same cell, the first application of a chimeric CRISPR construct consisting of a spacer from one species in the context of repeats of another species, and the first report of a potential impact of CRISPR on genome-scale evolution by interference with an essential gene.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

