Self-assembled nanoplatform for targeted delivery of chemotherapy agents via affinity-regulated molecular interactions
- PMID: 20667589
- PMCID: PMC2925180
- DOI: 10.1016/j.biomaterials.2010.06.038
Self-assembled nanoplatform for targeted delivery of chemotherapy agents via affinity-regulated molecular interactions
Abstract
Site-specific delivery of drugs while minimizing unwanted distribution has been one of the pursued goals in cancer therapy. In this endeavor, we have developed targeted polymeric nanoparticles called amphiphilic urethane acrylate nonionomer (UAN) for encapsulation of diverse water-insoluble drugs and diagnostic agents, as well as for simple and reproducible surface conjugation of targeting ligands. Using monoclonal antibodies or lymphocyte function-associated antigen-1 (LFA-1) I domain engineered for varying affinities to intercellular adhesion molecule (ICAM)-1, we were able to deliver UAN nanoparticles to human cancer cells with the efficiency dependent on the strength of the molecular interactions and the degree of ICAM-1 expression on cell surface. Compared to non-specific uptake of free drugs, targeted delivery of UAN nanoparticles carrying equal amount of drugs produced more potent cytotoxicity. Notably, without the targeting ligands attached, UAN nanoparticles were largely precluded from non-specific uptake by the cells, resulting in much lower toxicity. The versatility of our UAN nanoparticles in both payload encapsulation and presentation of targeting ligands may facilitate developing a robust platform for evaluating various combinations of cancer drugs and molecular interactions toward developing effective cancer therapy formulations.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

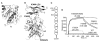

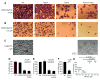
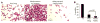


Similar articles
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Development of l-Tyrosine-Based Enzyme-Responsive Amphiphilic Poly(ester-urethane) Nanocarriers for Multiple Drug Delivery to Cancer Cells.Biomacromolecules. 2017 Jan 9;18(1):189-200. doi: 10.1021/acs.biomac.6b01476. Epub 2016 Dec 8. Biomacromolecules. 2017. PMID: 28064504
-
Self-Assembled Nanocarriers Based on Amphiphilic Natural Polymers for Anti- Cancer Drug Delivery Applications.Curr Pharm Des. 2017;23(35):5213-5229. doi: 10.2174/1381612823666170526111029. Curr Pharm Des. 2017. PMID: 28552068 Review.
-
Development of l-Amino-Acid-Based Hydroxyl Functionalized Biodegradable Amphiphilic Polyesters and Their Drug Delivery Capabilities to Cancer Cells.Biomacromolecules. 2020 Jan 13;21(1):171-187. doi: 10.1021/acs.biomac.9b01124. Epub 2019 Oct 22. Biomacromolecules. 2020. PMID: 31592651
-
Tumor suppression via paclitaxel-loaded drug carriers that target inflammation marker upregulated in tumor vasculature and macrophages.Biomaterials. 2013 Jan;34(2):598-605. doi: 10.1016/j.biomaterials.2012.10.004. Epub 2012 Oct 23. Biomaterials. 2013. PMID: 23099063
Cited by
-
Site-Specific Modification of Single-Chain Antibody Fragments for Bioconjugation and Vascular Immunotargeting.Bioconjug Chem. 2018 Jan 17;29(1):56-66. doi: 10.1021/acs.bioconjchem.7b00592. Epub 2017 Dec 29. Bioconjug Chem. 2018. PMID: 29200285 Free PMC article.
-
Inflamed leukocyte-mimetic nanoparticles for molecular imaging of inflammation.Biomaterials. 2011 Oct;32(30):7651-61. doi: 10.1016/j.biomaterials.2011.06.030. Epub 2011 Jul 23. Biomaterials. 2011. PMID: 21783245 Free PMC article.
-
Chitosan-Alginate Microcapsules Provide Gastric Protection and Intestinal Release of ICAM-1-Targeting Nanocarriers, Enabling GI Targeting In Vivo.Adv Funct Mater. 2016 May 24;26(20):3382-3393. doi: 10.1002/adfm.201600084. Epub 2016 Apr 23. Adv Funct Mater. 2016. PMID: 27375374 Free PMC article.
-
ICAM-1 Targeted Drug Combination Nanoparticles Enhanced Gemcitabine-Paclitaxel Exposure and Breast Cancer Suppression in Mouse Models.Pharmaceutics. 2021 Dec 31;14(1):89. doi: 10.3390/pharmaceutics14010089. Pharmaceutics. 2021. PMID: 35056985 Free PMC article.
-
Distinct subcellular trafficking resulting from monomeric vs multimeric targeting to endothelial ICAM-1: implications for drug delivery.Mol Pharm. 2014 Dec 1;11(12):4350-62. doi: 10.1021/mp500409y. Epub 2014 Oct 24. Mol Pharm. 2014. PMID: 25301142 Free PMC article.
References
-
- Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–71. - PubMed
-
- Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–30. - PubMed
-
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. - PubMed
-
- Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004;5:449–55. - PubMed
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

