Activation of phenylalanine hydroxylase induces positive cooperativity toward the natural cofactor
- PMID: 20667834
- PMCID: PMC2945563
- DOI: 10.1074/jbc.M110.124016
Activation of phenylalanine hydroxylase induces positive cooperativity toward the natural cofactor
Abstract
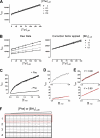
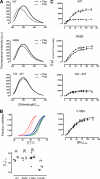
Protein misfolding with loss-of-function of the enzyme phenylalanine hydroxylase (PAH) is the molecular basis of phenylketonuria in many individuals carrying missense mutations in the PAH gene. PAH is complexly regulated by its substrate L-Phenylalanine and its natural cofactor 6R-L-erythro-5,6,7,8-tetrahydrobiopterin (BH(4)). Sapropterin dihydrochloride, the synthetic form of BH(4), was recently approved as the first pharmacological chaperone to correct the loss-of-function phenotype. However, current knowledge about enzyme function and regulation in the therapeutic setting is scarce. This illustrates the need for comprehensive analyses of steady state kinetics and allostery beyond single residual enzyme activity determinations to retrace the structural impact of missense mutations on the phenylalanine hydroxylating system. Current standard PAH activity assays are either indirect (NADH) or discontinuous due to substrate and product separation before detection. We developed an automated fluorescence-based continuous real-time PAH activity assay that proved to be faster and more efficient but as precise and accurate as standard methods. Wild-type PAH kinetic analyses using the new assay revealed cooperativity of activated PAH toward BH(4), a previously unknown finding. Analyses of structurally preactivated variants substantiated BH(4)-dependent cooperativity of the activated enzyme that does not rely on the presence of l-Phenylalanine but is determined by activating conformational rearrangements. These findings may have implications for an individualized therapy, as they support the hypothesis that the patient's metabolic state has a more significant effect on the interplay of the drug and the conformation and function of the target protein than currently appreciated.
Figures

Similar articles
-
The interplay between genotype, metabolic state and cofactor treatment governs phenylalanine hydroxylase function and drug response.Hum Mol Genet. 2011 Jul 1;20(13):2628-41. doi: 10.1093/hmg/ddr165. Epub 2011 Apr 28. Hum Mol Genet. 2011. PMID: 21527427
-
Loss of function in phenylketonuria is caused by impaired molecular motions and conformational instability.Am J Hum Genet. 2008 Jul;83(1):5-17. doi: 10.1016/j.ajhg.2008.05.013. Epub 2008 Jun 5. Am J Hum Genet. 2008. PMID: 18538294 Free PMC article.
-
New insights into tetrahydrobiopterin pharmacodynamics from Pah enu1/2, a mouse model for compound heterozygous tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency.Biochem Pharmacol. 2010 Nov 15;80(10):1563-71. doi: 10.1016/j.bcp.2010.07.042. Epub 2010 Aug 10. Biochem Pharmacol. 2010. PMID: 20705059
-
Phenylalanine hydroxylase: function, structure, and regulation.IUBMB Life. 2013 Apr;65(4):341-9. doi: 10.1002/iub.1150. Epub 2013 Mar 4. IUBMB Life. 2013. PMID: 23457044 Review.
-
Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency, state of the art.Mol Genet Metab. 2003 Feb;78(2):93-9. doi: 10.1016/s1096-7192(02)00229-9. Mol Genet Metab. 2003. PMID: 12618080 Review.
Cited by
-
Manipulation of a cation-π sandwich reveals conformational flexibility in phenylalanine hydroxylase.Biochimie. 2021 Apr;183:63-77. doi: 10.1016/j.biochi.2020.11.011. Epub 2020 Nov 19. Biochimie. 2021. PMID: 33221376 Free PMC article.
-
Queuosine deficiency in eukaryotes compromises tyrosine production through increased tetrahydrobiopterin oxidation.J Biol Chem. 2011 Jun 3;286(22):19354-63. doi: 10.1074/jbc.M111.219576. Epub 2011 Apr 12. J Biol Chem. 2011. PMID: 21487017 Free PMC article.
-
Long-term follow-up of patients with phenylketonuria treated with tetrahydrobiopterin: a seven years experience.Orphanet J Rare Dis. 2015 Feb 8;10:14. doi: 10.1186/s13023-015-0227-8. Orphanet J Rare Dis. 2015. PMID: 25757997 Free PMC article. Clinical Trial.
-
In Silico and In Vitro Tailoring of a Chitosan Nanoformulation of a Human Metabolic Enzyme.Pharmaceutics. 2021 Mar 4;13(3):329. doi: 10.3390/pharmaceutics13030329. Pharmaceutics. 2021. PMID: 33806405 Free PMC article.
-
Structural features of the regulatory ACT domain of phenylalanine hydroxylase.PLoS One. 2013 Nov 14;8(11):e79482. doi: 10.1371/journal.pone.0079482. eCollection 2013. PLoS One. 2013. PMID: 24244510 Free PMC article.
References
-
- Zschocke J. (2003) Hum. Mutat. 21, 345–356 - PubMed
-
- Fiege B., Blau N. (2007) J. Pediatr. 150, 627–630 - PubMed
-
- Muntau A. C., Röschinger W., Habich M., Demmelmair H., Hoffmann B., Sommerhoff C. P., Roscher A. A. (2002) N. Engl. J. Med. 347, 2122–2132 - PubMed
-
- Levy H. L., Milanowski A., Chakrapani A., Cleary M., Lee P., Trefz F. K., Whitley C. B., Feillet F., Feigenbaum A. S., Bebchuk J. D., Christ-Schmidt H., Dorenbaum A. (2007) Lancet 370, 504–510 - PubMed
-
- Trefz F. K., Burton B. K., Longo N., Casanova M. M., Gruskin D. J., Dorenbaum A., Kakkis E. D., Crombez E. A., Grange D. K., Harmatz P., Lipson M. H., Milanowski A., Randolph L. M., Vockley J., Whitley C. B., Wolff J. A., Bebchuk J., Christ-Schmidt H., Hennermann J. B. (2009) J. Pediatr. 154, 700–707 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

