A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer
- PMID: 20667969
- PMCID: PMC3078712
- DOI: 10.1634/theoncologist.2010-0030
A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer
Abstract
Introduction: Bevacizumab is increasingly being tested with neoadjuvant regimens in patients with localized cancer, but its effects on metastasis and survival remain unknown. This study examines the long-term outcome of clinical stage II/III rectal cancer patients treated in a prospective phase II study of bevacizumab with chemoradiation and surgery. As a benchmark, we used data from an analysis of 42 patients with locally advanced rectal cancer treated with a contemporary approach of preoperative fluoropyrimidine-based radiation therapy.
Materials and methods: Outcome analyses were performed on 32 patients treated prospectively with neoadjuvant bevacizumab, 5-fluorouracil, radiation therapy, and surgery as well as 42 patients treated with standard fluoropyrimidine-based chemoradiation.
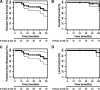
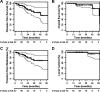
Results: Overall survival, disease-free survival, and local control showed favorable trends in patients treated with bevacizumab with chemoradiation followed by surgery. Acute and postoperative toxicity appeared acceptable.
Conclusions: Neoadjuvant bevacizumab with standard chemoradiation and surgery shows promising long-term efficacy and safety profiles in locally advanced rectal cancer patients.
Conflict of interest statement
This article discusses unlabeled, investigational, or alternative use of bevacizumab with radiotherapy in the treatment of rectal cancer.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures
References
-
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

