Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcome with O(6)-methylguanine methyltransferase (MGMT) promoter methylation and expression
- PMID: 20668043
- PMCID: PMC5393383
- DOI: 10.1210/jc.2010-0441
Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcome with O(6)-methylguanine methyltransferase (MGMT) promoter methylation and expression
Abstract
Context: The typically indolent behavior of pituitary tumors is juxtaposed with high rates of tumor cell invasion into adjacent dural structures, and occasional aggressive behavior. Although clinically significant invasion and malignant transformation remain uncommon, there are limited treatment options available for the management of these aggressive tumors. Recently, case reports have described efficacy of temozolomide for the treatment of aggressive pituitary tumors.
Design: Seven patients with aggressive pituitary tumors have been treated with temozolomide. We compared O(6)-methylguanine methyltransferase (MGMT) promoter methylation and MGMT expression in 14 surgical specimens from these seven patients and correlated these molecular features with the clinical response to temozolomide.
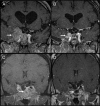
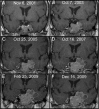
Results: Significant tumor regression was seen in two patients (29%), a 20% reduction in tumor volume with subsequent stable tumor size was noted in one patient, arrest of tumor growth occurred in three patients, and progressive metastatic disease developed during treatment in one patient. The DNA promoter site for MGMT was unmethylated in all 14 adequate specimens, and variable MGMT expression was seen in all 14 cases. There was no correlation between MGMT expression and clinical outcomes.
Conclusions: We conclude that medical therapy with temozolomide can be helpful in the management of life-threatening pituitary tumors that have failed to respond to conventional treatments. The optimal duration of treatment in patients with stabilization or reduction of tumor size has not been established, and long-term follow up studies are needed.
Figures


References
-
- Meij BP, Lopes MB, Ellegala DB, Alden TD, Laws Jr ER 2002. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated by transsphenoidal surgery. J Neurosurg 96:195–208 - PubMed
-
- Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young Jr WF, Lloyd RV, Davis DH, Guthrie BL, Schoene WC 1997. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer 79:804–812 - PubMed
-
- Melmed S, Sternberg R, Cook D, Klibanski A, Chanson P, Bonert V, Vance ML, Rhew D, Kleinberg D, Barkan A 2005. A critical analysis of tumor shrinkage during primary medical therapy in acromegaly. J Clin Endocrinol Metab 90:4405–4410 - PubMed
-
- Buchfelder M, Weigel D, Droste M, Mann K, Saller B, Brübach K, Stalla GK, Bidlingmaier M, Strasburger CJ 2009. Investigators of German Pegvisomant Observational Study. Pituitary tumor size in acromegaly during pegvisomant treatment: experience from MR re-evaluations of the German Pegvisomant Observational Study. Eur J Endocrinol 161:27–35 - PubMed
-
- Batista DL, Zhang X, Gejman R, Ansell PJ, Zhou Y, Johnson SA, Swearingen B, Hedley-Whyte ET, Stratakis CA, Klibanski A 2006. The effects of SOM230 on cell proliferation and adrenocorticotropin secretion in human corticotroph pituitary adenomas. J Clin Endocrinol Metab 91:4482–4488 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

