Interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunction
- PMID: 20668055
- PMCID: PMC2957783
- DOI: 10.1124/jpet.110.172262
Interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunction
Abstract
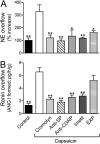
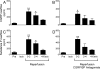
Renin, the rate-limiting enzyme in the activation of the renin-angiotensin system (RAS), is synthesized and stored in cardiac mast cells. In ischemia/reperfusion, cardiac sensory nerves release neuropeptides such as substance P that, by degranulating mast cells, might promote renin release, thus activating a local RAS and ultimately inducing cardiac dysfunction. We tested this hypothesis in whole hearts ex vivo, in cardiac nerve terminals in vitro, and in cultured mast cells. We found that substance P-containing nerves are juxtaposed to renin-containing cardiac mast cells. Chemical stimulation of these nerves elicited substance P release that was accompanied by renin release, with the latter being preventable by mast cell stabilization or blockade of substance P receptors. Substance P caused degranulation of mast cells in culture and elicited renin release, and both of these were prevented by substance P receptor blockade. Ischemia/reperfusion in ex vivo hearts caused the release of substance P, which was associated with an increase in renin and norepinephrine overflow and with sustained reperfusion arrhythmias; substance P receptor blockade prevented these changes. Substance P, norepinephrine, and renin were also released by acetaldehyde, a known product of ischemia/reperfusion, from cardiac synaptosomes and cultured mast cells, respectively. Collectively, our findings indicate that an important link exists in the heart between sensory nerves and renin-containing mast cells; substance P released from sensory nerves plays a significant role in the release of mast cell renin in ischemia/reperfusion and in the activation of a local cardiac RAS. This culminates in angiotensin production, norepinephrine release, and arrhythmic cardiac dysfunction.
Figures






Similar articles
-
Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells.Circulation. 2010 Aug 24;122(8):771-81. doi: 10.1161/CIRCULATIONAHA.110.952481. Epub 2010 Aug 9. Circulation. 2010. PMID: 20697027 Free PMC article.
-
Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion.J Clin Invest. 2006 Apr;116(4):1063-70. doi: 10.1172/JCI25713. J Clin Invest. 2006. PMID: 16585966 Free PMC article.
-
Histamine H4-receptors inhibit mast cell renin release in ischemia/reperfusion via protein kinase C ε-dependent aldehyde dehydrogenase type-2 activation.J Pharmacol Exp Ther. 2014 Jun;349(3):508-17. doi: 10.1124/jpet.114.214122. Epub 2014 Apr 2. J Pharmacol Exp Ther. 2014. PMID: 24696042 Free PMC article.
-
Targeting cardiac mast cells: pharmacological modulation of the local renin-angiotensin system.Curr Pharm Des. 2011 Nov;17(34):3744-52. doi: 10.2174/138161211798357908. Curr Pharm Des. 2011. PMID: 22103845 Free PMC article. Review.
-
Salvaging the Ischemic Heart: Gi-Coupled Receptors in Mast Cells Activate a PKCε/ALDH2 Pathway Providing Anti-RAS Cardioprotection.Curr Med Chem. 2018;25(34):4416-4431. doi: 10.2174/0929867325666180214115127. Curr Med Chem. 2018. PMID: 29446730 Review.
Cited by
-
Remodeling of intrinsic cardiac neurons: effects of β-adrenergic receptor blockade in guinea pig models of chronic heart disease.Am J Physiol Regul Integr Comp Physiol. 2012 Nov 1;303(9):R950-8. doi: 10.1152/ajpregu.00223.2012. Epub 2012 Aug 29. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22933026 Free PMC article.
-
Histamine 3 receptor activation reduces the expression of neuronal angiotensin II type 1 receptors in the heart.J Pharmacol Exp Ther. 2012 Jan;340(1):185-91. doi: 10.1124/jpet.111.187765. Epub 2011 Oct 19. J Pharmacol Exp Ther. 2012. PMID: 22011436 Free PMC article.
-
Ambroxol for the treatment of fibromyalgia: science or fiction?J Pain Res. 2017 Aug 16;10:1905-1929. doi: 10.2147/JPR.S139223. eCollection 2017. J Pain Res. 2017. PMID: 28860846 Free PMC article.
-
Aldehyde dehydrogenase type 2 activation by adenosine and histamine inhibits ischemic norepinephrine release in cardiac sympathetic neurons: mediation by protein kinase Cε.J Pharmacol Exp Ther. 2012 Oct;343(1):97-105. doi: 10.1124/jpet.112.196626. Epub 2012 Jul 3. J Pharmacol Exp Ther. 2012. PMID: 22761303 Free PMC article.
-
Regulation of mast cell responses in health and disease.Crit Rev Immunol. 2011;31(6):475-529. doi: 10.1615/critrevimmunol.v31.i6.30. Crit Rev Immunol. 2011. PMID: 22321108 Free PMC article. Review.
References
-
- Bader M. (2002) Role of the local renin-angiotensin system in cardiac damage: a minireview focussing on transgenic animal models. J Mol Cell Cardiol 34:1455–1462 - PubMed
-
- Barlucchi L, Leri A, Dostal DE, Fiordaliso F, Tada H, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. (2001) Canine ventricular myocytes possess a renin-angiotensin system that is upregulated with heart failure. Circ Res 88:298–304 - PubMed
-
- Bauer O, Razin E. (2000) Mast cell-nerve interactions. News Physiol Sci 15:213–218 - PubMed
-
- Camici PG, Pagani M. (2006) Cardiac nociception. Circulation 114:2309–2312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

