Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids
- PMID: 20668104
- PMCID: PMC2957252
- DOI: 10.1152/ajprenal.00196.2010
Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids
Abstract
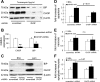
Apoptosis of podocytes is considered critical in the pathogenesis of diabetic nephropathy (DN). Free fatty acids (FFAs) are critically involved in the pathogenesis of diabetes mellitus type 2, in particular the regulation of pancreatic β cell survival. The objectives of this study were to elucidate the role of palmitic acid, palmitoleic, and oleic acid in the regulation of podocyte cell death and endoplasmic reticulum (ER) stress. We show that palmitic acid increases podocyte cell death, both apoptosis and necrosis of podocytes, in a dose and time-dependent fashion. Palmitic acid induces podocyte ER stress, leading to an unfolded protein response as reflected by the induction of the ER chaperone immunoglobulin heavy chain binding protein (BiP) and proapoptotic C/EBP homologous protein (CHOP) transcription factor. Of note, the monounsaturated palmitoleic and oleic acid can attenuate the palmitic acid-induced upregulation of CHOP, thereby preventing cell death. Similarly, gene silencing of CHOP protects against palmitic acid-induced podocyte apoptosis. Our results offer a rationale for interventional studies aimed at testing whether dietary shifting of the FFA balance toward unsaturated FFAs can delay the progression of DN.
Figures




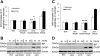
Similar articles
-
Inhibitory effect of unsaturated fatty acids on saturated fatty acid-induced apoptosis in human pancreatic β-cells: activation of caspases and ER stress induction.Cell Physiol Biochem. 2011;27(5):525-38. doi: 10.1159/000329954. Epub 2011 Jun 15. Cell Physiol Biochem. 2011. PMID: 21691070
-
Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2.Am J Pathol. 2013 Sep;183(3):735-44. doi: 10.1016/j.ajpath.2013.05.023. Epub 2013 Jul 16. Am J Pathol. 2013. PMID: 23867797 Free PMC article.
-
Oleate rescues INS-1E β-cells from palmitate-induced apoptosis by preventing activation of the unfolded protein response.Biochem Biophys Res Commun. 2013 Nov 29;441(4):770-6. doi: 10.1016/j.bbrc.2013.10.130. Epub 2013 Nov 1. Biochem Biophys Res Commun. 2013. PMID: 24189472
-
CHOP and the endoplasmic reticulum stress response in myelinating glia.Curr Opin Neurobiol. 2009 Oct;19(5):505-10. doi: 10.1016/j.conb.2009.08.007. Epub 2009 Sep 8. Curr Opin Neurobiol. 2009. PMID: 19744850 Free PMC article. Review.
-
Fatty Acids: An Insight into the Pathogenesis of Neurodegenerative Diseases and Therapeutic Potential.Int J Mol Sci. 2022 Feb 25;23(5):2577. doi: 10.3390/ijms23052577. Int J Mol Sci. 2022. PMID: 35269720 Free PMC article. Review.
Cited by
-
Endoplasmic-reticulum-stress-induced lipotoxicity in human kidney epithelial cells.Toxicol Res (Camb). 2022 Jul 22;11(4):683-695. doi: 10.1093/toxres/tfac041. eCollection 2022 Aug. Toxicol Res (Camb). 2022. PMID: 36051659 Free PMC article.
-
Hypergravity Load Modulates Acetaminophen Nephrotoxicity via Endoplasmic Reticulum Stress in Association with Hepatic microRNA-122 Expression.Int J Mol Sci. 2021 May 5;22(9):4901. doi: 10.3390/ijms22094901. Int J Mol Sci. 2021. PMID: 34063126 Free PMC article.
-
Acetyl Co-A Carboxylase Inhibition Halts Hyperglycemia Induced Upregulation of De Novo Lipogenesis in Podocytes and Proximal Tubular Cells.Metabolites. 2022 Oct 3;12(10):940. doi: 10.3390/metabo12100940. Metabolites. 2022. PMID: 36295842 Free PMC article.
-
Rhizoma coptidis as a Potential Treatment Agent for Type 2 Diabetes Mellitus and the Underlying Mechanisms: A Review.Front Pharmacol. 2019 Jul 22;10:805. doi: 10.3389/fphar.2019.00805. eCollection 2019. Front Pharmacol. 2019. PMID: 31396083 Free PMC article. Review.
-
Targeted and untargeted metabolomic approach for GDM diagnosis.Front Mol Biosci. 2023 Jan 5;9:997436. doi: 10.3389/fmolb.2022.997436. eCollection 2022. Front Mol Biosci. 2023. PMID: 36685282 Free PMC article.
References
-
- USRDS The United States Renal Data System. Am J Kidney Dis 42: 1–230, 2003. - PubMed
-
- Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep 6: 177–181, 2006 - PubMed
-
- Chen HM, Liu ZH, Zeng CH, Li SJ, Wang QW, Li LS. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis 48: 772–779, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

